Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2
- PMID: 22719267
- PMCID: PMC3375257
- DOI: 10.1371/journal.pgen.1002760
Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2
Abstract
Cells respond to defects in mitochondrial function by activating signaling pathways that restore homeostasis. The mitochondrial peptide exporter HAF-1 and the bZip transcription factor ATFS-1 represent one stress response pathway that regulates the transcription of mitochondrial chaperone genes during mitochondrial dysfunction. Here, we report that GCN-2, an eIF2α kinase that modulates cytosolic protein synthesis, functions in a complementary pathway to that of HAF-1 and ATFS-1. During mitochondrial dysfunction, GCN-2-dependent eIF2α phosphorylation is required for development as well as the lifespan extension observed in Caenorhabditis elegans. Reactive oxygen species (ROS) generated from dysfunctional mitochondria are required for GCN-2-dependent eIF2α phosphorylation but not ATFS-1 activation. Simultaneous deletion of ATFS-1 and GCN-2 compounds the developmental defects associated with mitochondrial stress, while stressed animals lacking GCN-2 display a greater dependence on ATFS-1 and stronger induction of mitochondrial chaperone genes. These findings are consistent with translational control and stress-dependent chaperone induction acting in complementary arms of the UPR(mt).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
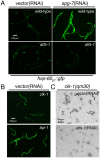




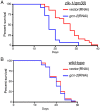
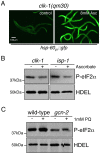
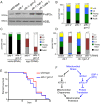
References
-
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. - PubMed
-
- Baker BM, Haynes CM. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem Sci. 2011;36:254–261. - PubMed
-
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. - PubMed
-
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

