5-(4-Fluoro-phen-yl)-3-[5-methyl-1-(4-methyl-phen-yl)-1H-1,2,3-triazol-4-yl]-4,5-dihydro-1H-pyrazole-1-carbothio-amide
- PMID: 22719703
- PMCID: PMC3379505
- DOI: 10.1107/S1600536812024245
5-(4-Fluoro-phen-yl)-3-[5-methyl-1-(4-methyl-phen-yl)-1H-1,2,3-triazol-4-yl]-4,5-dihydro-1H-pyrazole-1-carbothio-amide
Abstract
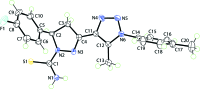
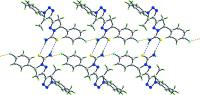
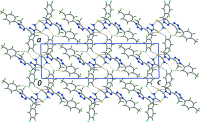
In the title compound, C(20)H(19)FN(6)S, the pyrazole ring has an envelope conformation, with the methine C atom being the flap atom. The dihedral angle between the least-squares plane through the pyrazole and triazole rings is 7.59 (9)°, and the triazole and attached benzene ring form a dihedral angle of 74.79 (9)°. The thio-urea group is coplanar with the pyrazole ring [N-N-C-S torsion angle = -179.93 (11)°], which enables the formation of an intra-molecular N-H⋯N hydrogen bond. In the crystal, inversion-related mol-ecules associate via N-H⋯S hydrogen bonds and eight-membered {⋯HNCS}(2) synthons feature in the crystal packing. These synthons are connected into supra-molecular chains along the a axis via N-H⋯F hydrogen bonds, and the chains are consolidated into layers in the ab plane via C-H⋯S and C-H⋯F contacts.
Figures
References
-
- Abdel-Wahab, B. F., Abdel-Latif, E., Mohamed, H. A. & Awad, G. E. A. (2012a). Eur. J. Med. Chem. 52, 263–268. - PubMed
-
- Agilent (2011). CrysAlis PRO Agilent Technologies, Yarnton, Oxfordshire, England.
-
- Booth, J. H. & Ross, A. S. (1982). US Patent 4336375.
-
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
