Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial
- PMID: 22719796
- PMCID: PMC3377180
- DOI: 10.4338/ACI-2012-03-RA-0009
Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial
Abstract
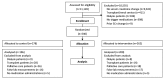
OBJECTIVES: Clinical decision support (CDS), such as computerized alerts, improves prescribing in the setting of acute kidney injury (AKI), but considerable opportunity remains to improve patient safety. The authors sought to determine whether pharmacy surveillance of AKI patients could detect and prevent medication errors that are not corrected by automated interventions. METHODS: The authors conducted a randomized clinical trial among 396 patients admitted to an academic, tertiary care hospital between June 1, 2010 and August 31, 2010 with an acute 0.5 mg/dl change in serum creatinine over 48 hours and a nephrotoxic or renally cleared medication order. Patients randomly assigned to the intervention group received surveillance from a clinical pharmacist using a web-based surveillance tool to monitor drug prescribing and kidney function trends. CDS alerting and standard pharmacy services were active in both study arms. Outcome measures included blinded adjudication of potential adverse drug events (pADEs), adverse drug events (ADEs) and time to provider modification or discontinuation of targeted nephrotoxic or renally cleared medications. RESULTS: Potential ADEs or ADEs occurred for 104 (8.0%) of control and 99 (7.1%) of intervention patient-medication pairs (p=0.4). Additionally, the time to provider modification or discontinuation of targeted nephrotoxic or renally cleared medications did not differ between control and intervention patients (33.4 hrs vs. 30.3 hrs, p=0.3). CONCLUSIONS: Pharmacy surveillance had no incremental benefit over previously implemented CDS alerts.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

