The physiology and proteomics of drought tolerance in maize: early stomatal closure as a cause of lower tolerance to short-term dehydration?
- PMID: 22719860
- PMCID: PMC3374823
- DOI: 10.1371/journal.pone.0038017
The physiology and proteomics of drought tolerance in maize: early stomatal closure as a cause of lower tolerance to short-term dehydration?
Abstract
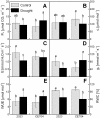
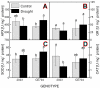
Understanding the response of a crop to drought is the first step in the breeding of tolerant genotypes. In our study, two maize (Zea mays L.) genotypes with contrasting sensitivity to dehydration were subjected to moderate drought conditions. The subsequent analysis of their physiological parameters revealed a decreased stomatal conductance accompanied by a slighter decrease in the relative water content in the sensitive genotype. In contrast, the tolerant genotype maintained open stomata and active photosynthesis, even under dehydration conditions. Drought-induced changes in the leaf proteome were analyzed by two independent approaches, 2D gel electrophoresis and iTRAQ analysis, which provided compatible but only partially overlapping results. Drought caused the up-regulation of protective and stress-related proteins (mainly chaperones and dehydrins) in both genotypes. The differences in the levels of various detoxification proteins corresponded well with the observed changes in the activities of antioxidant enzymes. The number and levels of up-regulated protective proteins were generally lower in the sensitive genotype, implying a reduced level of proteosynthesis, which was also indicated by specific changes in the components of the translation machinery. Based on these results, we propose that the hypersensitive early stomatal closure in the sensitive genotype leads to the inhibition of photosynthesis and, subsequently, to a less efficient synthesis of the protective/detoxification proteins that are associated with drought tolerance.
Conflict of interest statement
Figures





References
-
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29:185– 212.
-
- Pinheiro C, Chaves MM. Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot. 2011;62:869– 882. - PubMed
-
- Chernyad’ev II. Effect of water stress on the photosynthetic apparatus of plants and the protective role of cytokinins: A review. Appl Biochem Microbiol. 2005;41:115– 128. - PubMed
-
- Pospisilova J, Vagner M, Malbeck J, Travniakova A, Batkova P. Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol Plant. 2005;49:533– 540.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

