Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK--analysis of national data
- PMID: 22719897
- PMCID: PMC3374836
- DOI: 10.1371/journal.pone.0038504
Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK--analysis of national data
Abstract
Background: Delay in implementing new treatments into clinical practice results in considerable health and economic opportunity costs. Data from the UK TOBY Cooling Register provides the opportunity to examine how one new effective therapy for newborn infants suspected of suffering asphyxial encephalopathy--therapeutic hypothermia- was implemented in the UK.
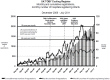
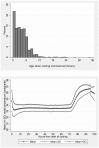
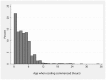
Methodology/principal findings: We analysed returned data forms from inception of the Register in December 2006 to the end of July 2011. Data forms were received for 1384 (67%) of the 2069 infants registered. The monthly rate of notifications increased from median {IQR} 18 {15-31} to 33 {30-39} after the announcement of the results of the recent TOBY trial, and to 50 {36-55} after their publication. This rate further increased to 70 {64-83} following official endorsement of the therapy, and is now close to the expected numbers of eligible infants. Cooling was started at 3.3 {1.5-5.5} hours after birth and the time taken to achieve the target 33-34 °C rectal temperature was 1 {0-3} hours. The rectal temperature was in the target range in 83% of measurements. From 2006 to 2011 there was evidence of extension of treatment to slightly less severely affected infants. 278 of 1362 (20%) infants died at 2.9 {1.4-4.1} days of age. The rates of death fell slightly over the period of the Register and, at two years of age cerebral palsy was diagnosed in 22% of infants; half of these were spastic bilateral. Factors independently associated with adverse outcome were clinical seizures prior to cooling (p<0.001) and severely abnormal amplitude integrated EEG (p<0.001).
Conclusions/significance: Therapeutic hypothermia was implemented appropriately within the UK, with significant benefit to patients and the health economy. This may be due in part to participation by neonatal units in clinical trials, the establishment of the national Register, and its endorsement by advisory bodies.
Conflict of interest statement
Figures



References
-
- Cooksey D. 2006. A review of UK health research funding http://www.hm-treasury.gov.uk Available: http://www.hm-treasury.gov.uk/d/pbr06_cooksey_final_report_636.pdf Accessed: 2012 May 15.
-
- Gunn AJ, Battin M, Gluckman PD, Gunn TR, Bennet L. Therapeutic hypothermia: from lab to NICU. J Perinat Med. 2005;33:340–346. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. - PubMed
-
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. - PubMed
-
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

