FRET-based localization of fluorescent protein insertions within the ryanodine receptor type 1
- PMID: 22719904
- PMCID: PMC3374828
- DOI: 10.1371/journal.pone.0038594
FRET-based localization of fluorescent protein insertions within the ryanodine receptor type 1
Abstract
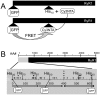
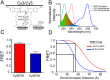
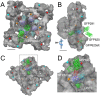
Fluorescent protein (FP) insertions have often been used to localize primary structure elements in mid-resolution 3D cryo electron microscopic (EM) maps of large protein complexes. However, little is known as to the precise spatial relationship between the location of the fused FP and its insertion site within a larger protein. To gain insights into these structural considerations, Förster resonance energy transfer (FRET) measurements were used to localize green fluorescent protein (GFP) insertions within the ryanodine receptor type 1 (RyR1), a large intracellular Ca(2+) release channel that plays a key role in skeletal muscle excitation contraction coupling. A series of full-length His-tagged GFP-RyR1 fusion constructs were created, expressed in human embryonic kidney (HEK)-293T cells and then complexed with Cy3NTA, a His-tag specific FRET acceptor. FRET efficiency values measured from each GFP donor to Cy3NTA bound to each His tag acceptor site were converted into intermolecular distances and the positions of each inserted GFP were then triangulated relative to a previously published X-ray crystal structure of a 559 amino acid RyR1 fragment. We observed that the chromophoric centers of fluorescent proteins inserted into RyR1 can be located as far as 45 Å from their insertion sites and that the fused proteins can also be located in internal cavities within RyR1. These findings should prove useful in interpreting structural results obtained in cryo EM maps using fusions of small fluorescent proteins. More accurate point-to-point distance information may be obtained using complementary orthogonal labeling systems that rely on fluorescent probes that bind directly to amino acid side chains.
Conflict of interest statement
Figures



Similar articles
-
N-terminal and central segments of the type 1 ryanodine receptor mediate its interaction with FK506-binding proteins.J Biol Chem. 2013 May 31;288(22):16073-84. doi: 10.1074/jbc.M113.463299. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585572 Free PMC article.
-
Förster resonance energy transfer measurements of ryanodine receptor type 1 structure using a novel site-specific labeling method.PLoS One. 2009 Oct 12;4(10):e7338. doi: 10.1371/journal.pone.0007338. PLoS One. 2009. PMID: 19823671 Free PMC article.
-
Site-specific labeling of the type 1 ryanodine receptor using biarsenical fluorophores targeted to engineered tetracysteine motifs.PLoS One. 2013 May 28;8(5):e64686. doi: 10.1371/journal.pone.0064686. Print 2013. PLoS One. 2013. PMID: 23724080 Free PMC article.
-
Localization of the dantrolene-binding sequence near the FK506-binding protein-binding site in the three-dimensional structure of the ryanodine receptor.J Biol Chem. 2011 Apr 8;286(14):12202-12. doi: 10.1074/jbc.M110.194316. Epub 2011 Jan 24. J Biol Chem. 2011. PMID: 21262961 Free PMC article.
-
Regulatory mechanisms of ryanodine receptor/Ca2+ release channel revealed by recent advancements in structural studies.J Muscle Res Cell Motil. 2021 Jun;42(2):291-304. doi: 10.1007/s10974-020-09575-6. Epub 2020 Feb 10. J Muscle Res Cell Motil. 2021. PMID: 32040690 Free PMC article. Review.
Cited by
-
N-terminal and central segments of the type 1 ryanodine receptor mediate its interaction with FK506-binding proteins.J Biol Chem. 2013 May 31;288(22):16073-84. doi: 10.1074/jbc.M113.463299. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585572 Free PMC article.
-
Molecular-scale GPS: positioning a biosensor peptide on RyR.Biophys J. 2014 Nov 4;107(9):2003-5. doi: 10.1016/j.bpj.2014.09.028. Biophys J. 2014. PMID: 25418085 Free PMC article. No abstract available.
-
Two regions of the ryanodine receptor calcium channel are involved in Ca(2+)-dependent inactivation.Biochemistry. 2014 Mar 4;53(8):1373-9. doi: 10.1021/bi401586h. Epub 2014 Feb 21. Biochemistry. 2014. PMID: 24521037 Free PMC article.
-
Fluorescence Resonance Energy Transfer-based Structural Analysis of the Dihydropyridine Receptor α1S Subunit Reveals Conformational Differences Induced by Binding of the β1a Subunit.J Biol Chem. 2016 Jun 24;291(26):13762-70. doi: 10.1074/jbc.M115.704049. Epub 2016 Apr 25. J Biol Chem. 2016. PMID: 27129199 Free PMC article.
-
Structural mapping of divergent regions in the type 1 ryanodine receptor using fluorescence resonance energy transfer.Structure. 2014 Sep 2;22(9):1322-1332. doi: 10.1016/j.str.2014.07.003. Epub 2014 Aug 14. Structure. 2014. PMID: 25132084 Free PMC article.
References
-
- Charpilienne A, Nejmeddine M, Berois M, Parez N, Neumann E, et al. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J Biol Chem. 2001;276:29361–29367. - PubMed
-
- Liu Z, Zhang J, Wang R, Wayne ChenSR, Wagenknecht T. Location of divergent region 2 on the three-dimensional structure of cardiac muscle ryanodine receptor/calcium release channel. J Mol Biol. 2004;338:533–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

