Antibiotic transport in resistant bacteria: synchrotron UV fluorescence microscopy to determine antibiotic accumulation with single cell resolution
- PMID: 22719907
- PMCID: PMC3373604
- DOI: 10.1371/journal.pone.0038624
Antibiotic transport in resistant bacteria: synchrotron UV fluorescence microscopy to determine antibiotic accumulation with single cell resolution
Abstract
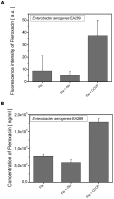
A molecular definition of the mechanism conferring bacterial multidrug resistance is clinically crucial and today methods for quantitative determination of the uptake of antimicrobial agents with single cell resolution are missing. Using the naturally occurring fluorescence of antibacterial agents after deep ultraviolet (DUV) excitation, we developed a method to non-invasively monitor the quinolones uptake in single bacteria. Our approach is based on a DUV fluorescence microscope coupled to a synchrotron beamline providing tuneable excitation from 200 to 600 nm. A full spectrum was acquired at each pixel of the image, to study the DUV excited fluorescence emitted from quinolones within single bacteria. Measuring spectra allowed us to separate the antibiotic fluorescence from the autofluorescence contribution. By performing spectroscopic analysis, the quantification of the antibiotic signal was possible. To our knowledge, this is the first time that the intracellular accumulation of a clinical antibiotic could be determined and discussed in relation with the level of drug susceptibility for a multiresistant strain. This method is especially important to follow the behavior of quinolone molecules at individual cell level, to quantify the intracellular concentration of the antibiotic and develop new strategies to combat the dissemination of MDR-bacteria. In addition, this original approach also indicates the heterogeneity of bacterial population when the same strain is under environmental stress like antibiotic attack.
Conflict of interest statement
Figures
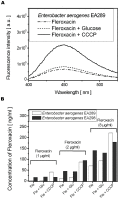
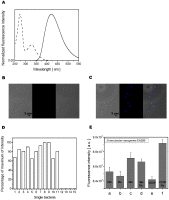
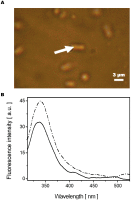
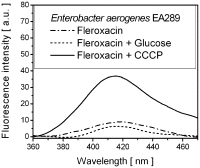
References
-
- de Kraker MEA, Davey PG, Grundmann H on behalf of the BURDEN study group. Mortality and Hospital Stay Associated with Resistant Staphylococcus aureus and Escherichia coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PloS Med 8(10): e1001104. doi:10.1371/journal.pmed.1001104. 2011. - PMC - PubMed
-
- Rice LB. Emerging issues in the management of infections caused by multidrug-resistant gram-negative bacteria. Cleve Clin J Med. 2007;74:S12–20. - PubMed
-
- Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29:630–636. - PubMed
-
- Blot S, Depuydt P, Vandewoude K, De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis. 2007;20:391–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

