Respiratory insufficiency correlated strongly with mortality of rodents infected with West Nile virus
- PMID: 22719920
- PMCID: PMC3375279
- DOI: 10.1371/journal.pone.0038672
Respiratory insufficiency correlated strongly with mortality of rodents infected with West Nile virus
Abstract
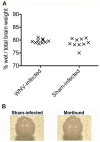
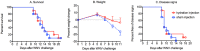
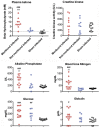
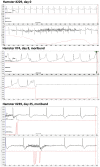
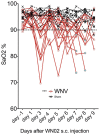
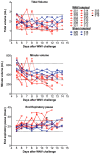
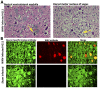
West Nile virus (WNV) disease can be fatal for high-risk patients. Since WNV or its antigens have been identified in multiple anatomical locations of the central nervous system of persons or rodent models, one cannot know where to investigate the actual mechanism of mortality without careful studies in animal models. In this study, depressed respiratory functions measured by plethysmography correlated strongly with mortality. This respiratory distress, as well as reduced oxygen saturation, occurred beginning as early as 4 days before mortality. Affected medullary respiratory control cells may have contributed to the animals' respiratory insufficiency, because WNV antigen staining was present in neurons located in the ventrolateral medulla. Starvation or dehydration would be irrelevant in people, but could cause death in rodents due to lethargy or loss of appetite. Animal experiments were performed to exclude this possibility. Plasma ketones were increased in moribund infected hamsters, but late-stage starvation markers were not apparent. Moreover, daily subcutaneous administration of 5% dextrose in physiological saline solution did not improve survival or other disease signs. Therefore, infected hamsters did not die from starvation or dehydration. No cerebral edema was apparent in WNV- or sham-infected hamsters as determined by comparing wet-to-total weight ratios of brains, or by evaluating blood-brain-barrier permeability using Evans blue dye penetration into brains. Limited vasculitis was present in the right atrium of the heart of infected hamsters, but abnormal electrocardiograms for several days leading up to mortality did not occur. Since respiratory insufficiency was strongly correlated with mortality more than any other pathological parameter, it is the likely cause of death in rodents. These animal data and a poor prognosis for persons with respiratory insufficiency support the hypothesis that neurological lesions affecting respiratory function may be the primary cause of human WNV-induced death.
Conflict of interest statement
Figures

References
-
- Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease - United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. - PubMed
-
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, et al. Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res. 2004;63:41–50. - PubMed
-
- Leis AA, Stokic DS. Neuromuscular Manifestations of Human West Nile Virus Infection. Curr Treat Options Neurol. 2005;7:15–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

