A randomised controlled trial of two infusion rates to decrease reactions to antivenom
- PMID: 22719932
- PMCID: PMC3377702
- DOI: 10.1371/journal.pone.0038739
A randomised controlled trial of two infusion rates to decrease reactions to antivenom
Abstract
Background: Snake envenoming is a major clinical problem in Sri Lanka, with an estimated 40,000 bites annually. Antivenom is only available from India and there is a high rate of systemic hypersensitivity reactions. This study aimed to investigate whether the rate of infusion of antivenom reduced the frequency of severe systemic hypersensitivity reactions.
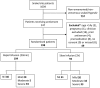
Methods and findings: This was a randomized comparison trial of two infusion rates of antivenom for treatment of non-pregnant adult patients (>14 y) with snake envenoming in Sri Lanka. Snake identification was by patient or hospital examination of dead snakes when available and confirmed by enzyme-immunoassay for Russell's viper envenoming. Patients were blindly allocated in a 11 randomisation schedule to receive antivenom either as a 20 minute infusion (rapid) or a two hour infusion (slow). The primary outcome was the proportion with severe systemic hypersensitivity reactions (grade 3 by Brown grading system) within 4 hours of commencement of antivenom. Secondary outcomes included the proportion with mild/moderate hypersensitivity reactions and repeat antivenom doses. Of 1004 patients with suspected snakebites, 247 patients received antivenom. 49 patients were excluded or not recruited leaving 104 patients allocated to the rapid antivenom infusion and 94 to the slow antivenom infusion. The median actual duration of antivenom infusion in the rapid group was 20 min (Interquartile range[IQR]:20-25 min) versus 120 min (IQR:75-120 min) in the slow group. There was no difference in severe systemic hypersensitivity reactions between those given rapid and slow infusions (32% vs. 35%; difference 3%; 95%CI:-10% to +17%;p = 0.65). The frequency of mild/moderate reactions was also similar. Similar numbers of patients in each arm received further doses of antivenom (30/104 vs. 23/94).
Conclusions: A slower infusion rate would not reduce the rate of severe systemic hypersensitivity reactions from current high rates. More effort should be put into developing better quality antivenoms.
Trial registration: www.slctr.lk SLCTR/2007/005.
Conflict of interest statement
Figures


References
-
- Lalloo DG, Theakston RD. Snake antivenoms. Journal of Toxicology: Clinical Toxicology. . 2003;41(3):277–90. - PubMed
-
- Gawarammana IB, Kularatne SAM, Dissanayake WP, Kumarasiri RP, Senanayake N, et al. Parallel infusion of hydrocortisone +/− chlorpheniramine bolus injection to prevent acute adverse reactions to antivenom for snakebites - a randomised, double-blind, placebo-controlled study. Medical Journal of Australia. . 2004;180(1):20–3. - PubMed
-
- Isbister GK, Brown SG, MacDonald E, White J, Currie BJ. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Medical Journal of Australia. . 2008;188(8):473–6. - PubMed
-
- Habib AG. Effect of pre-medication on early adverse reactions following antivenom use in snakebite: a systematic review and meta-analysis. Drug Saf. . 2011;34(10):869–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

