Regulation of classical cadherin membrane expression and F-actin assembly by alpha-catenins, during Xenopus embryogenesis
- PMID: 22719936
- PMCID: PMC3374811
- DOI: 10.1371/journal.pone.0038756
Regulation of classical cadherin membrane expression and F-actin assembly by alpha-catenins, during Xenopus embryogenesis
Abstract
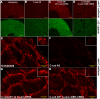
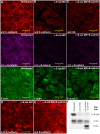
Alpha (α)-E-catenin is a component of the cadherin complex, and has long been thought to provide a link between cell surface cadherins and the actin skeleton. More recently, it has also been implicated in mechano-sensing, and in the control of tissue size. Here we use the early Xenopus embryos to explore functional differences between two α-catenin family members, α-E- and α-N-catenin, and their interactions with the different classical cadherins that appear as tissues of the embryo become segregated from each other. We show that they play both cadherin-specific and context-specific roles in the emerging tissues of the embryo. α-E-catenin interacts with both C- and E-cadherin. It is specifically required for junctional localization of C-cadherin, but not of E-cadherin or N-cadherin at the neurula stage. α-N-cadherin interacts only with, and is specifically required for junctional localization of, N-cadherin. In addition, α -E-catenin is essential for normal tissue size control in the non-neural ectoderm, but not in the neural ectoderm or the blastula. We also show context specificity in cadherin/ α-catenin interactions. E-cadherin requires α-E-catenin for junctional localization in some tissues, but not in others, during early development. These specific functional cadherin/alpha-catenin interactions may explain the basis of cadherin specificity of actin assembly and morphogenetic movements seen previously in the neural and non-neural ectoderm.
Conflict of interest statement
Figures









References
-
- Takeichi M, Hatta K, Nose A, Nagafuchi A. Identification of a gene family of cadherin cell adhesion molecules. Cell Differ Dev. 1988;25 - PubMed
-
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–108. - PubMed
-
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. - PubMed
-
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

