Maternal melatonin programs the daily pattern of energy metabolism in adult offspring
- PMID: 22719949
- PMCID: PMC3373595
- DOI: 10.1371/journal.pone.0038795
Maternal melatonin programs the daily pattern of energy metabolism in adult offspring
Abstract
Background: Shift work was recently described as a factor that increases the risk of Type 2 diabetes mellitus. In addition, rats born to mothers subjected to a phase shift throughout pregnancy are glucose intolerant. However, the mechanism by which a phase shift transmits metabolic information to the offspring has not been determined. Among several endocrine secretions, phase shifts in the light/dark cycle were described as altering the circadian profile of melatonin production by the pineal gland. The present study addresses the importance of maternal melatonin for the metabolic programming of the offspring.
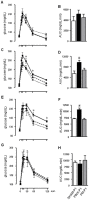
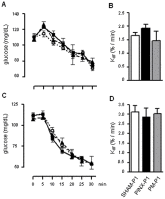
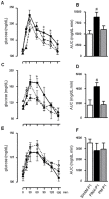
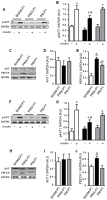
Methodology/principal findings: Female Wistar rats were submitted to SHAM surgery or pinealectomy (PINX). The PINX rats were divided into two groups and received either melatonin (PM) or vehicle. The SHAM, the PINX vehicle and the PM females were housed with male Wistar rats. Rats were allowed to mate and after weaning, the male and female offspring were subjected to a glucose tolerance test (GTT), a pyruvate tolerance test (PTT) and an insulin tolerance test (ITT). Pancreatic islets were isolated for insulin secretion, and insulin signaling was assessed in the liver and in the skeletal muscle by western blots. We found that male and female rats born to PINX mothers display glucose intolerance at the end of the light phase of the light/dark cycle, but not at the beginning. We further demonstrate that impaired glucose-stimulated insulin secretion and hepatic insulin resistance are mechanisms that may contribute to glucose intolerance in the offspring of PINX mothers. The metabolic programming described here occurs due to an absence of maternal melatonin because the offspring born to PINX mothers treated with melatonin were not glucose intolerant.
Conclusions/significance: The present results support the novel concept that maternal melatonin is responsible for the programming of the daily pattern of energy metabolism in their offspring.
Conflict of interest statement
Figures


References
-
- Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23:155–168. - PubMed
-
- Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011;96:E1862–E1865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

