Lack of evidence for neonatal misoprostol neurodevelopmental toxicity in C57BL6/J mice
- PMID: 22719983
- PMCID: PMC3374803
- DOI: 10.1371/journal.pone.0038911
Lack of evidence for neonatal misoprostol neurodevelopmental toxicity in C57BL6/J mice
Abstract
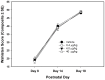
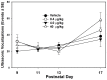
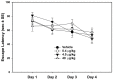
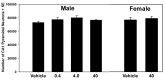
Misoprostol is a synthetic analogue of prostaglandin E1 that is administered to women at high doses to induce uterine contractions for early pregnancy termination and at low doses to aid in cervical priming during labor. Because of the known teratogenic effects of misoprostol when given during gestation and its effects on axonal growth in vitro, we examined misoprostol for its potential as a neurodevelopmental toxicant when administered to neonatal C57BL6/J mice. Mice were injected subcutaneously (s.c.) with 0.4, 4 or 40 µg/kg misoprostol on postnatal day 7, the approximate developmental stage in mice of human birth, after which neonatal somatic growth, and sensory and motor system development were assessed. These doses were selected to span the range of human exposure used to induce labor. In addition, adult mice underwent a battery of behavioral tests relevant to neurodevelopmental disorders such as autism including tests for anxiety, stereotyped behaviors, social communication and interactions, and learning and memory. No significant effects of exposure were found for any measure of development or behavioral endpoints. In conclusion, the results of the present study in C57BL/6J mice do not provide support for neurodevelopmental toxicity after misoprostol administration approximating human doses and timed to coincide with the developmental stage of human birth.
Conflict of interest statement
Figures
Similar articles
-
Vaginal misoprostol for pre-abortion cervical priming: is there an optimal evacuation time interval?Br J Obstet Gynaecol. 1999 Mar;106(3):266-9. doi: 10.1111/j.1471-0528.1999.tb08241.x. Br J Obstet Gynaecol. 1999. PMID: 10426647 Clinical Trial.
-
Congenital abnormalities in Brazilian children associated with misoprostol misuse in first trimester of pregnancy.Lancet. 1998 May 30;351(9116):1624-7. doi: 10.1016/S0140-6736(97)12363-7. Lancet. 1998. PMID: 9620717
-
Uterine Scar Dehiscence Associated with Misoprostol Cervical Priming for Surgical Abortion: A Case Report.J Reprod Med. 2015 Sep-Oct;60(9-10):445-8. J Reprod Med. 2015. PMID: 26592074
-
[Developmental toxicity of misoprostol: an update].Rev Med Chil. 2011 Apr;139(4):516-23. Epub 2011 Aug 25. Rev Med Chil. 2011. PMID: 21879192 Review. Spanish.
-
Methotrexate/misoprostol embryopathy: report of four cases resulting from failed medical abortion.Am J Med Genet A. 2003 Nov 15;123A(1):72-8. doi: 10.1002/ajmg.a.20503. Am J Med Genet A. 2003. PMID: 14556250 Review.
Cited by
-
Misoprostol Reverse Hippocampal Neuron Cyclooxygenase-2 Downstream Signaling Imbalance in Aluminum-Overload Rats.Curr Alzheimer Res. 2016;13(9):1006-16. doi: 10.2174/1567205013666160401114601. Curr Alzheimer Res. 2016. PMID: 27033056 Free PMC article.
References
-
- Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–786. - PubMed
-
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–3099. - PubMed
-
- Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, et al. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics. 2001;108:1155–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

