Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta
- PMID: 22720036
- PMCID: PMC3375250
- DOI: 10.1371/journal.pone.0039080
Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta
Abstract
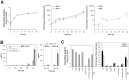
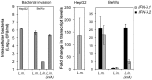
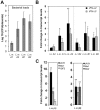
Bacterial infections trigger the expression of type I and II interferon genes but little is known about their effect on type III interferon (IFN-λ) genes, whose products play important roles in epithelial innate immunity against viruses. Here, we studied the expression of IFN-λ genes in cultured human epithelial cells infected with different pathogenic bacteria and in the mouse placenta infected with Listeria monocytogenes. We first showed that in intestinal LoVo cells, induction of IFN-λ genes by L. monocytogenes required bacterial entry and increased further during the bacterial intracellular phase of infection. Other Gram-positive bacteria, Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis, also induced IFN-λ genes when internalized by LoVo cells. In contrast, Gram-negative bacteria Salmonella enterica serovar Typhimurium, Shigella flexneri and Chlamydia trachomatis did not substantially induce IFN-λ. We also found that IFN-λ genes were up-regulated in A549 lung epithelial cells infected with Mycobacterium tuberculosis and in HepG2 hepatocytes and BeWo trophoblastic cells infected with L. monocytogenes. In a humanized mouse line permissive to fetoplacental listeriosis, IFN-λ2/λ3 mRNA levels were enhanced in placentas infected with L. monocytogenes. In addition, the feto-placental tissue was responsive to IFN-λ2. Together, these results suggest that IFN-λ may be an important modulator of the immune response to Gram-positive intracellular bacteria in epithelial tissues.
Conflict of interest statement
Figures


References
-
- Mordstein M, Michiels T, Staeheli P. What have we learned from the IL28 receptor knockout mouse? Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2010;30:579–584. - PubMed
-
- Witte K, Witte E, Sabat R, Wolk K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine & growth factor reviews. 2010;21:237–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

