Zinc-finger antiviral protein inhibits XMRV infection
- PMID: 22720057
- PMCID: PMC3376128
- DOI: 10.1371/journal.pone.0039159
Zinc-finger antiviral protein inhibits XMRV infection
Abstract
Background: The zinc-finger antiviral protein (ZAP) is a host factor that specifically inhibits the replication of certain viruses, including Moloney murine leukemia virus (MoMLV), HIV-1, and certain alphaviruses and filoviruses. ZAP binds to specific viral mRNAs and recruits cellular mRNA degradation machinery to degrade the target RNA. The common features of ZAP-responsive RNA sequences remain elusive and thus whether a virus is susceptible to ZAP can only be determined experimentally. Xenotropic murine leukemia virus-related virus (XMRV) is a recently identified γ-retrovirus that was originally thought to be involved in prostate cancer and chronic fatigue syndrome but recently proved to be a laboratory artefact. Nonetheless, XMRV as a new retrovirus has been extensively studied. Since XMRV and MoMLV share only 67.9% sequence identity in the 3'UTRs, which is the target sequence of ZAP in MoMLV, whether XMRV is susceptible to ZAP remains to be determined.
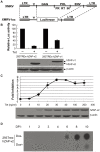
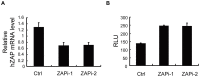
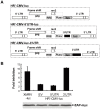
Findings: We constructed an XMRV-luc vector, in which the coding sequences of Gag-Pol and part of Env were replaced with luciferase-coding sequence. Overexpression of ZAP potently inhibited the expression of XMRV-luc in a ZAP expression-level-dependent manner, while downregulation of endogenous ZAP rendered cells more sensitive to infection. Furthermore, ZAP inhibited the spreading of replication-competent XMRV. Consistent with the previously reported mechanisms by which ZAP inhibits viral infection, ZAP significantly inhibited the accumulation of XMRV-luc mRNA in the cytoplasm. The ZAP-responsive element in XMRV mRNA was mapped to the 3'UTR.
Conclusions: ZAP inhibits XMRV replication by preventing the accumulation of viral mRNA in the cytoplasm. Documentation of ZAP inhibiting XMRV helps to broaden the spectrum of ZAP's antiviral activity. Comparison of the target sequences of ZAP in XMRV and MoMLV helps to better understand the features of ZAP-responsive elements.
Conflict of interest statement
Figures



Similar articles
-
Antiviral Activity of Zinc Finger Antiviral Protein (ZAP) in Different Virus Families.Pathogens. 2023 Dec 17;12(12):1461. doi: 10.3390/pathogens12121461. Pathogens. 2023. PMID: 38133344 Free PMC article. Review.
-
Versatility of the Zinc-Finger Antiviral Protein (ZAP) As a Modulator of Viral Infections.Int J Biol Sci. 2024 Aug 26;20(12):4585-4600. doi: 10.7150/ijbs.98029. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309436 Free PMC article. Review.
-
Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation.Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15834-9. doi: 10.1073/pnas.1101676108. Epub 2011 Aug 29. Proc Natl Acad Sci U S A. 2011. PMID: 21876179 Free PMC article.
-
TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein.J Virol. 2017 Apr 13;91(9):e00088-17. doi: 10.1128/JVI.00088-17. Print 2017 May 1. J Virol. 2017. PMID: 28202764 Free PMC article.
-
Intrinsic DNA synthesis fidelity of xenotropic murine leukemia virus-related virus reverse transcriptase.FEBS J. 2012 Apr;279(8):1433-44. doi: 10.1111/j.1742-4658.2012.08532.x. Epub 2012 Mar 16. FEBS J. 2012. PMID: 22340433 Free PMC article.
Cited by
-
Riplet Binds the Zinc Finger Antiviral Protein (ZAP) and Augments ZAP-Mediated Restriction of HIV-1.J Virol. 2022 Aug 24;96(16):e0052622. doi: 10.1128/jvi.00526-22. Epub 2022 Aug 1. J Virol. 2022. PMID: 35913217 Free PMC article.
-
Antiviral Activity of Zinc Finger Antiviral Protein (ZAP) in Different Virus Families.Pathogens. 2023 Dec 17;12(12):1461. doi: 10.3390/pathogens12121461. Pathogens. 2023. PMID: 38133344 Free PMC article. Review.
-
MIV-150 and zinc acetate combination provides potent and broad activity against HIV-1.Drug Deliv Transl Res. 2017 Dec;7(6):859-866. doi: 10.1007/s13346-017-0421-4. Drug Deliv Transl Res. 2017. PMID: 28812250
-
Versatility of the Zinc-Finger Antiviral Protein (ZAP) As a Modulator of Viral Infections.Int J Biol Sci. 2024 Aug 26;20(12):4585-4600. doi: 10.7150/ijbs.98029. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309436 Free PMC article. Review.
-
Characterization of Novel Splice Variants of Zinc Finger Antiviral Protein (ZAP).J Virol. 2019 Aug 28;93(18):e00715-19. doi: 10.1128/JVI.00715-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31118263 Free PMC article.
References
-
- Gao G, Guo X, Goff SP. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science. 2002;297:1703–1706. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

