Fangchinoline inhibits human immunodeficiency virus type 1 replication by interfering with gp160 proteolytic processing
- PMID: 22720080
- PMCID: PMC3374765
- DOI: 10.1371/journal.pone.0039225
Fangchinoline inhibits human immunodeficiency virus type 1 replication by interfering with gp160 proteolytic processing
Abstract
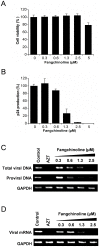
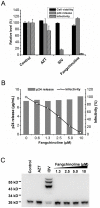
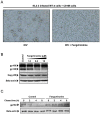
The introduction of highly active antiretroviral therapy has led to a significant reduction in the morbidity and mortality of acquired immunodeficiency syndrome patients. However, the emergence of drug resistance has resulted in the failure of treatments in large numbers of patients and thus necessitates the development of new classes of anti-HIV drugs. In this study, more than 200 plant-derived small-molecule compounds were evaluated in a cell-based HIV-1 antiviral screen, resulting in the identification of a novel HIV-1 inhibitor (fangchinoline). Fangchinoline, a bisbenzylisoquinoline alkaloid isolated from Radix Stephaniae tetrandrae, exhibited antiviral activity against HIV-1 laboratory strains NL4-3, LAI and BaL in MT-4 and PM1 cells with a 50% effective concentration ranging from 0.8 to 1.7 µM. Mechanism-of-action studies showed that fangchinoline did not exhibit measurable antiviral activity in TZM-b1 cells but did inhibit the production of infectious virions in HIV-1 cDNA transfected 293T cells, which suggests that the compound targets a late event in infection cycle. Furthermore, the antiviral effect of fangchinoline seems to be HIV-1 envelope-dependent, as the production of infectious HIV-1 particles packaged with a heterologous envelope, the vesicular stomatitis virus G glycoprotein, was unaffected by fangchinoline. Western blot analysis of HIV envelope proteins expressed in transfected 293T cells and in isolated virions showed that fangchinoline inhibited HIV-1 gp160 processing, resulting in reduced envelope glycoprotein incorporation into nascent virions. Collectively, our results demonstrate that fangchinoline inhibits HIV-1 replication by interfering with gp160 proteolytic processing. Fangchinoline may serve as a starting point for developing a new HIV-1 therapeutic approach.
Conflict of interest statement
Figures


Similar articles
-
Identification and characterization of UK-201844, a novel inhibitor that interferes with human immunodeficiency virus type 1 gp160 processing.Antimicrob Agents Chemother. 2007 Oct;51(10):3554-61. doi: 10.1128/AAC.00643-07. Epub 2007 Jul 23. Antimicrob Agents Chemother. 2007. PMID: 17646410 Free PMC article.
-
Analysis of Select Herpes Simplex Virus 1 (HSV-1) Proteins for Restriction of Human Immunodeficiency Virus Type 1 (HIV-1): HSV-1 gM Protein Potently Restricts HIV-1 by Preventing Intracellular Transport and Processing of Env gp160.J Virol. 2018 Jan 2;92(2):e01476-17. doi: 10.1128/JVI.01476-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093081 Free PMC article.
-
Megalomicin inhibits HIV-1 replication and interferes with gp160 processing.Virology. 1997 Dec 22;239(2):303-14. doi: 10.1006/viro.1997.8872. Virology. 1997. PMID: 9434722
-
Proteolytic events of HIV-1 replication as targets for therapeutic intervention.Curr Pharm Des. 2003;9(22):1803-15. doi: 10.2174/1381612033454478. Curr Pharm Des. 2003. PMID: 12871198 Review.
-
Maturation of HIV envelope glycoprotein precursors by cellular endoproteases.Biochim Biophys Acta. 2000 Nov 10;1469(3):121-32. doi: 10.1016/s0304-4157(00)00014-9. Biochim Biophys Acta. 2000. PMID: 11063880 Review.
Cited by
-
Fangchinoline Inhibits African Swine Fever Virus Replication by Suppressing the AKT/mTOR/NF-κB Signaling Pathway in Porcine Alveolar Macrophages.Int J Mol Sci. 2024 Jun 29;25(13):7178. doi: 10.3390/ijms25137178. Int J Mol Sci. 2024. PMID: 39000284 Free PMC article.
-
Five new secondary metabolites produced by a marine-associated fungus, Daldinia eschscholzii.Mar Drugs. 2014 Nov 20;12(11):5563-75. doi: 10.3390/md12115563. Mar Drugs. 2014. PMID: 25419997 Free PMC article.
-
Fangchinoline inhibits growth and biofilm of Candida albicans by inducing ROS overproduction.J Cell Mol Med. 2024 May;28(9):e18354. doi: 10.1111/jcmm.18354. J Cell Mol Med. 2024. PMID: 38686557 Free PMC article.
-
anti-HCoV: A web resource to collect natural compounds against human coronaviruses.Trends Food Sci Technol. 2020 Dec;106:1-11. doi: 10.1016/j.tifs.2020.09.007. Epub 2020 Sep 22. Trends Food Sci Technol. 2020. PMID: 32982062 Free PMC article. Review.
-
Alkaloids: Therapeutic Potential against Human Coronaviruses.Molecules. 2020 Nov 24;25(23):5496. doi: 10.3390/molecules25235496. Molecules. 2020. PMID: 33255253 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

