Oxygen tension is a determinant of the matrix-forming phenotype of cultured human meniscal fibrochondrocytes
- PMID: 22720095
- PMCID: PMC3376130
- DOI: 10.1371/journal.pone.0039339
Oxygen tension is a determinant of the matrix-forming phenotype of cultured human meniscal fibrochondrocytes
Abstract
Background: Meniscal cartilage displays a poor repair capacity, especially when injury is located in the avascular region of the tissue. Cell-based tissue engineering strategies to generate functional meniscus substitutes is a promising approach to treat meniscus injuries. Meniscus fibrochondrocytes (MFC) can be used in this approach. However, MFC are unable to retain their phenotype when expanded in culture. In this study, we explored the effect of oxygen tension on MFC expansion and on their matrix-forming phenotype.
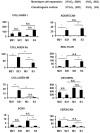
Methodology/principal findings: MFC were isolated from human menisci followed by basic fibroblast growth factor (FGF-2) mediated cell expansion in monolayer culture under normoxia (21%O(2)) or hypoxia (3%O(2)). Normoxia and hypoxia expanded MFC were seeded on to a collagen scaffold. The MFC seeded scaffolds (constructs) were cultured in a serum free chondrogenic medium for 3 weeks under normoxia and hypoxia. Constructs containing normoxia-expanded MFC were subsequently cultured under normoxia while those formed from hypoxia-expanded MFC were subsequently cultured under hypoxia. After 3 weeks of in vitro culture, the constructs were assessed biochemically, histologically and for gene expression via real-time reverse transcription-PCR assays. The results showed that constructs under normoxia produced a matrix with enhanced mRNA ratio (3.5-fold higher; p<0.001) of collagen type II to I. This was confirmed by enhanced deposition of collagen II using immuno-histochemistry. Furthermore, the constructs under hypoxia produced a matrix with higher mRNA ratio of aggrecan to versican (3.5-fold, p<0.05). However, both constructs had the same capacity to produce a glycosaminoglycan (GAG) -specific extracellular matrix.
Conclusions: Our data provide evidence that oxygen tension is a key player in determining the matrix phenotype of cultured MFC. These findings suggest that the use of normal and low oxygen tension during MFC expansion and subsequent neo-tissue formation cultures may be important in engineering different regions of the meniscus.
Conflict of interest statement
Figures




References
-
- Ahmed AM. The Load-Bearing Role of the Knee Meniscus. In: Knee Meniscus: Basic and Clinical Foundations. Edited by Van C Mow, Steven P Arnoczky, Douglas W Jackson. New York: Raven Press, Ltd. 1992. pp. 59–73.
-
- McDevitt CA, Miller RR, Spindler KP. The cells and cell matrix interactions of the meniscus. In: Knee Meniscus: Basic and Clinical Foundations. Edited by Van C Mow, Steven P Arnoczky, Douglas W Jackson. New York: Raven Press. 1992. pp. 29–36.
-
- McDevitt CA, Mukherjee S, Kambic H, Parker R. Emerging concepts of the cell biology of the meniscus. Curr Opin Orthop. 2002;13:345–350.
-
- Tanaka T, Fujii K, Kumagae Y. Comparison of biochemical characteristics of cultured fibrochondrocytes isolated from the inner and outer regions of human meniscus. Knee Surg Sports Traumatol Arthrosc. 1999;7:75–80. - PubMed
-
- Melrose J, Smith S, Cake M, Read R, Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

