A systematic analysis of experimental immunotherapies on tumors differing in size and duration of growth
- PMID: 22720238
- PMCID: PMC3377001
- DOI: 10.4161/onci.1.2.18311
A systematic analysis of experimental immunotherapies on tumors differing in size and duration of growth
Abstract
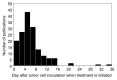
We conducted a systematic analysis to determine the reason for the apparent disparity of success of immunotherapy between clinical and experimental cancers. To do this, we performed a search of PubMed using the keywords "immunotherapy" AND "cancer" for the years of 1980 and 2010. The midspread of experimental tumors used in all the relevant literature published in 2010 were between 0.5-121 mm(3) in volume or had grown for four to eight days. Few studies reported large tumors that could be considered representative of clinical tumors, in terms of size and duration of growth. The predominant effect of cancer immunotherapies was slowed or delayed outgrowth. Regression of tumors larger than 200 mm(3) was observed only after passive antibody or adoptive T cell therapy. The effectiveness of other types of immunotherapy was generally scattered. By comparison, very few publications retrieved by the 1980 search could meet our selection criteria; all of these used tumors smaller than 100 mm(3), and none reported regression. In the entire year of 2010, only 13 used tumors larger than 400 mm(3), and nine of these reported tumor regression. Together, these results indicate that most recent studies, using many diverse approaches, still treat small tumors only to report slowed or delayed growth. Nevertheless, a few recent studies indicate effective therapy against large tumors when using passive antibody or adoptive T cell therapy. For the future, we aspire to witness the increased use of experimental studies treating tumors that model clinical cancers in terms of size and duration of growth.
Figures


References
-
- Woglom WH. Immunity to transplantable tumours. Cancer Rev. 1929;4:129–214.
-
- Weiss DW. Animal models of cancer immunotherapy: questions of relevance. Cancer Treat Rep. 1980;64:481–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
