Whole genome sequencing analysis of Plasmodium vivax using whole genome capture
- PMID: 22721170
- PMCID: PMC3410760
- DOI: 10.1186/1471-2164-13-262
Whole genome sequencing analysis of Plasmodium vivax using whole genome capture
Abstract
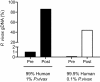
Background: Malaria caused by Plasmodium vivax is an experimentally neglected severe disease with a substantial burden on human health. Because of technical limitations, little is known about the biology of this important human pathogen. Whole genome analysis methods on patient-derived material are thus likely to have a substantial impact on our understanding of P. vivax pathogenesis and epidemiology. For example, it will allow study of the evolution and population biology of the parasite, allow parasite transmission patterns to be characterized, and may facilitate the identification of new drug resistance genes. Because parasitemias are typically low and the parasite cannot be readily cultured, on-site leukocyte depletion of blood samples is typically needed to remove human DNA that may be 1000X more abundant than parasite DNA. These features have precluded the analysis of archived blood samples and require the presence of laboratories in close proximity to the collection of field samples for optimal pre-cryopreservation sample preparation.
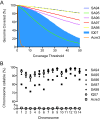
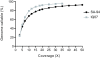
Results: Here we show that in-solution hybridization capture can be used to extract P. vivax DNA from human contaminating DNA in the laboratory without the need for on-site leukocyte filtration. Using a whole genome capture method, we were able to enrich P. vivax DNA from bulk genomic DNA from less than 0.5% to a median of 55% (range 20%-80%). This level of enrichment allows for efficient analysis of the samples by whole genome sequencing and does not introduce any gross biases into the data. With this method, we obtained greater than 5X coverage across 93% of the P. vivax genome for four P. vivax strains from Iquitos, Peru, which is similar to our results using leukocyte filtration (greater than 5X coverage across 96% ).
Conclusion: The whole genome capture technique will enable more efficient whole genome analysis of P. vivax from a larger geographic region and from valuable archived sample collections.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

