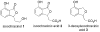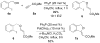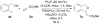Phosphine/palladium-catalyzed syntheses of alkylidene phthalans, 3-deoxyisoochracinic acid, isoochracinic acid, and isoochracinol
- PMID: 22721256
- PMCID: PMC3391320
- DOI: 10.1021/ol301154f
Phosphine/palladium-catalyzed syntheses of alkylidene phthalans, 3-deoxyisoochracinic acid, isoochracinic acid, and isoochracinol
Abstract
In this study we used sequential catalysis-PPh(3)--catalyzed nucleophilic addition followed by Pd(0)-catalyzed Heck cyclization--to construct complex functionalized alkylidene phthalans rapidly, in high yields, and with good stereoselectivities (E/Z ratios of up to 1:22). The scope of this Michael-Heck reaction includes substrates bearing various substituents around the alkylidene phthalan backbone. Applying this efficient sequential catalysis, we accomplished concise total syntheses of 3-deoxyisoochacinic acid, isoochracinic acid, and isoochracinol.
Figures
References
-
- Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2006.
-
-
For selected recent reviews on multicatalytic concepts, see: Ajamian A, Gleason JL. Angew Chem, Int Ed. 2004;43:3754.Lee JM, Na Y, Han H, Chang S. Chem Soc Rev. 2004;33:302.Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001.Enders D, Grondal C, Hüttl MRM. Angew Chem Int Ed. 2007;46:1570.Chapman CJ, Frost CG. Synthesis. 2007:1.Walji AM, MacMillan DWC. Synlett. 2007:1477.
-
-
-
Reviews of phosphine catalysis: Lu X, Zhang C, Xu Z. Acc Chem Res. 2001;34:535.Valentine DH, Jr, Hillhouse JH. Synthesis. 2003:317.Methot JL, Roush WR. Adv Synth Catal. 2004;346:1035.Lu X, Du Y, Lu C. Pure Appl Chem. 2005;77:1985.Nair V, Menon RS, Sreekanth AR, Abhilash N, Biju AT. Acc Chem Res. 2006;39:520.Ye LW, Zhou J, Tang Y. Chem Soc Rev. 2008;37:1140.Kwong CK-W, Fu MY, Lam CS-K, Toy PH. Synthesis. 2008:2307.Denmark SE, Beutner GL. Angew Chem Int Ed. 2008;47:1560.Ye LW, Zhou J, Tang Y. Chem Soc Rev. 2008;37:1140.Aroyan CE, Dermenci A, Miller SJ. Tetrahedron. 2009;65:4069.Kumara Swamy KC, Bhuvan Kumar NN, Balaraman E, Pavan Kumar KVP. Chem Rev. 2009;109:2551.Cowen BJ, Miller SJ. Chem Soc Rev. 2009;38:3102.Marinetti A, Voituriez A. Synlett. 2010:174.Kolesinska B. Cent Eur J Chem. 2010:1147.Wei Y, Shi M. Acc Chem Res. 2010;43:1005.Pinho e Melo TMVD. Monatsh Chem. 2011;142:681.Lalli C, Brioche J, Bernadat G, Masson G. Curr Org Chem. 2011;15:4108.Wang S-X, Han X, Zhong F, Wang Y, Lu Y. Synlett. 2011:2766.López F, Mascareñas JL. Chem Eur J. 2011;17:418.Zhao QY, Lian Z, Wei Y, Shi M. Chem Commun. 2012;48:1724.Fan YC, Kwon O. Phosphine Catalysis. In: List B, editor. Science of Synthesis, Asymmetric Organocatalysis, Vol 1, Lewis Base and Acid Catalysts. Georg Thieme; Stuttgart: 2012. pp. 723–782.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources