CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial
- PMID: 22721545
- PMCID: PMC3481366
- DOI: 10.1186/1745-6215-13-87
CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial
Abstract
Background: Worldwide, over 10 million people are killed or hospitalized because of traumatic brain injury each year. About 90% of deaths occur in low- and middle-income countries. The condition mostly affects young adults, and many experience long lasting or permanent disability. The social and economic burden is considerable. Tranexamic acid (TXA) is commonly given to surgical patients to reduce bleeding and the need for blood transfusion. It has been shown to reduce the number of patients receiving a blood transfusion by about a third, reduces the volume of blood transfused by about one unit, and halves the need for further surgery to control bleeding in elective surgical patients.
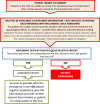
Methods/design: The CRASH-3 trial is an international, multicenter, pragmatic, randomized, double-blind, placebo-controlled trial to quantify the effects of the early administration of TXA on death and disability in patients with traumatic brain injury. Ten thousand adult patients who fulfil the eligibility criteria will be randomized to receive TXA or placebo. Adults with traumatic brain injury, who are within 8 h of injury and have any intracranial bleeding on computerized tomography (CT scan) or Glasgow Coma Score (GCS) of 12 or less can be included if the responsible doctor is substantially uncertain as to whether or not to use TXA in this patient. Patients with significant extracranial bleeding will be excluded since there is evidence that TXA improves outcome in these patients. Treatment will entail a 1 g loading dose followed by a 1 g maintenance dose over 8 h.The main analyses will be on an 'intention-to-treat' basis, irrespective of whether the allocated treatment was received. Results will be presented as appropriate effect estimates with a measure of precision (95% confidence intervals). Subgroup analyses for the primary outcome will be based on time from injury to randomization, the severity of the injury, location of the bleeding, and baseline risk. Interaction tests will be used to test whether the effect of treatment differs across these subgroups. A study with 10,000 patients will have approximately 90% power to detect a 15% relative reduction from 20% to 17% in all-cause mortality.
Trial registration: Current Controlled Trials ISRCTN15088122; Clinicaltrials.gov NCT01402882.
Figures
References
-
- Bruns J, Hauser W. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Supplement 10):2–10. - PubMed
-
- Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard University Press; 1996.
-
- Peden M, Scurfield R, Sleet D, Mohan D, Hyder A, Jarawan E, Mathers C. World Report on Road Traffic Injury Prevention. Geneva: World Health Organization; 2004.
-
- The CRASH-2 Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical