Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury
- PMID: 22721830
- PMCID: PMC3927911
- DOI: 10.1165/rcmb.2012-0090OC
Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury
Abstract
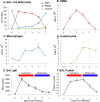
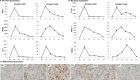
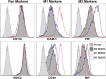
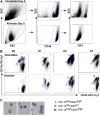
Macrophages are key orchestrators of the inflammatory and repair responses in the lung, and the diversity of their function is indicated by their polarized states and distinct subpopulations and localization in the lung. Here, we characterized the pulmonary macrophage populations in the interstitial and alveolar compartments during the induction and resolution of acute lung injury induced by Pseudomonas aeruginosa infection. We identified macrophage subpopulations and polarity according to FACS analysis of cell surface protein markers, combined with cell sorting for gene expression using real-time PCR. With these techniques, we validated a novel, alternatively activated (M2) marker (transferrin receptor), and we described three interstitial and alveolar macrophage subpopulations in the lung whose distribution and functional state evolved from the induction to resolution phases of lung injury. Together, these findings indicate the presence and evolution of distinct macrophage subsets in the lung that serve specific niches in regulating the inflammatory response and its resolution. Alterations in the balance and function of these subpopulations could lead to nonresolving acute lung injury.
Figures


Similar articles
-
Acute exposure to silica nanoparticles enhances mortality and increases lung permeability in a mouse model of Pseudomonas aeruginosa pneumonia.Part Fibre Toxicol. 2015 Jan 21;12(1):1. doi: 10.1186/s12989-014-0078-9. Part Fibre Toxicol. 2015. PMID: 25605549 Free PMC article.
-
Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas.Antimicrob Agents Chemother. 2010 Jun;54(6):2437-47. doi: 10.1128/AAC.01424-09. Epub 2010 Mar 15. Antimicrob Agents Chemother. 2010. PMID: 20231397 Free PMC article.
-
Extrathoracic multiple trauma dysregulates neutrophil function and exacerbates pneumonia-induced lung injury.J Trauma Acute Care Surg. 2021 Jun 1;90(6):924-934. doi: 10.1097/TA.0000000000003147. J Trauma Acute Care Surg. 2021. PMID: 34016916 Free PMC article.
-
Airway immunometabolites fuel Pseudomonas aeruginosa infection.Respir Res. 2020 Dec 10;21(1):326. doi: 10.1186/s12931-020-01591-x. Respir Res. 2020. PMID: 33302964 Free PMC article. Review.
-
Modulation of macrophage function for defence of the lung against Pseudomonas aeruginosa.Behring Inst Mitt. 1997 Feb;(98):274-82. Behring Inst Mitt. 1997. PMID: 9382751 Review.
Cited by
-
Primary graft dysfunction.Semin Respir Crit Care Med. 2013 Jun;34(3):305-319. doi: 10.1055/s-0033-1348474. Epub 2013 Jul 2. Semin Respir Crit Care Med. 2013. PMID: 23821506 Free PMC article. Review.
-
Recent advances in dead cell clearance during acute lung injury and repair.Fac Rev. 2021 Mar 30;10:33. doi: 10.12703/r/10-33. eCollection 2021. Fac Rev. 2021. PMID: 33977286 Free PMC article. Review.
-
The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases.Cells. 2021 Apr 14;10(4):897. doi: 10.3390/cells10040897. Cells. 2021. PMID: 33919784 Free PMC article. Review.
-
Mesenchymal stromal cells alleviate acute respiratory distress syndrome through the cholinergic anti-inflammatory pathway.Signal Transduct Target Ther. 2022 Sep 5;7(1):307. doi: 10.1038/s41392-022-01124-6. Signal Transduct Target Ther. 2022. PMID: 36064538 Free PMC article.
-
Initiation of LPS-induced pulmonary dysfunction and its recovery occur independent of T cells.BMC Pulm Med. 2018 Nov 22;18(1):174. doi: 10.1186/s12890-018-0741-2. BMC Pulm Med. 2018. PMID: 30466430 Free PMC article.
References
-
- Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J 2000;15:895–901 - PubMed
-
- Kotani M, Kotani T, Ishizaka A, Fujishima S, Koh H, Tasaka S, Sawafuji M, Ikeda E, Moriyama K, Kotake Y, et al. Neutrophil depletion attenuates interleukin-8 production in mild-overstretch ventilated normal rabbit lung. Crit Care Med 2004;32:514–519 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

