Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias
- PMID: 22721911
- PMCID: PMC3425771
- DOI: 10.1016/j.pain.2012.04.019
Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias
Erratum in
- Pain. 2012 Nov;153(11):2302
Abstract
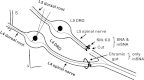
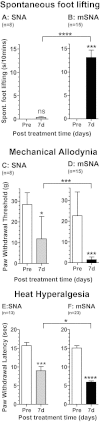
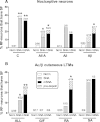
Partial nerve injury leads to peripheral neuropathic pain. This injury results in conducting/uninterrupted (also called uninjured)sensory fibres, conducting through the damaged nerve alongside axotomised/degenerating fibres. In rats seven days after L5 spinal nerve axotomy (SNA) or modified-SNA (added loose-ligation of L4 spinal nerve with neuroinflammation-inducing chromic-gut),we investigated (a) neuropathic pain behaviours and (b) electrophysiological changes in conducting/uninterrupted L4 dorsal root ganglion (DRG) neurons with receptive fields (called: L4-receptive-field-neurons). Compared to pretreatment, modified-SNA rats showed highly significant increases in spontaneous-foot lifting duration, mechanical-hypersensitivity/allodynia, and heathypersensitivity/hyperalgesia, that were significantly greater than after SNA, especially spontaneous-foot-lifting. We recorded intracellularly in vivo from normal L4/L5 DRG neurons and ipsilateral L4-receptive-field-neurons. After SNA or modified-SNA, L4-receptive-field-neurons showed the following: (a) increased percentages of C-, Aδ-, and Aβ-nociceptors and cutaneous Aα/β-low-thresholdmechanoreceptors with ongoing/spontaneous firing; (b) spontaneous firing in C-nociceptors that originated peripherally; this was ata faster rate in modified-SNA than SNA; (c) decreased electricalthresholds in A-nociceptors after SNA; (d) hyperpolarised membrane potentials in A-nociceptors and Aα/-low-thresholdmechanoreceptors after SNA, but not C-nociceptors; (e) decreased somatic action potential rise times in C- and A-nociceptors, not Aα/β-low-threshold-mechanoreceptors. We suggest that these changes in subtypes of conducting/uninterrupted neurons after partial nerve injury contribute to the different aspects of neuropathic pain as follows: spontaneous firing in nociceptors to ongoing/spontaneous pain; spontaneous firing in Aα/β-low-threshold-mechanoreceptors to dysesthesias/paresthesias; and lowered A-nociceptor electrical thresholds to A-nociceptor sensitization,and greater evoked pain [corrected].
Copyright © 2012 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Figures




Comment in
-
Afferent units and somatic sensation.Pain. 2012 Sep;153(9):1783-1784. doi: 10.1016/j.pain.2012.06.013. Epub 2012 Jul 25. Pain. 2012. PMID: 22835834 No abstract available.
References
-
- Abrahamsen B., Zhao J., Asante C.O., Cendan C.M., Marsh S., Martinez-Barbera J.P., Nassar M.A., Dickenson A.H., Wood J.N. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Amir R., Argoff C.E., Bennett G.J., Cummins T.R., Durieux M.E., Gerner P., Gold M.S., Porreca F., Strichartz G.R. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7:S1–S29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

