Dopamine reverses reward insensitivity in apathy following globus pallidus lesions
- PMID: 22721958
- PMCID: PMC3639369
- DOI: 10.1016/j.cortex.2012.04.013
Dopamine reverses reward insensitivity in apathy following globus pallidus lesions
Abstract
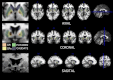
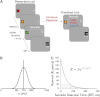
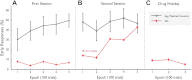
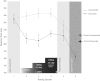
Apathy is a complex, behavioural disorder associated with reduced spontaneous initiation of actions. Although present in mild forms in some healthy people, it is a pathological state in conditions such as Alzheimer's and Parkinson's disease where it can have profoundly devastating effects. Understanding the mechanisms underlying apathy is therefore of urgent concern but this has proven difficult because widespread brain changes in neurodegenerative diseases make interpretation difficult and there is no good animal model. Here we present a very rare case with profound apathy following bilateral, focal lesions of the basal ganglia, with globus pallidus regions that connect with orbitofrontal (OFC) and ventromedial prefrontal cortex (VMPFC) particularly affected. Using two measures of oculomotor decision-making we show that apathy in this individual was associated with reward insensitivity. However, reward sensitivity could be established partially with levodopa and more effectively with a dopamine receptor agonist. Concomitantly, there was an improvement in the patient's clinical state, with reduced apathy, greater motivation and increased social interactions. These findings provide a model system to study a key neuropsychiatric disorder. They demonstrate that reward insensitivity associated with basal ganglia dysfunction might be an important component of apathy that can be reversed by dopaminergic modulation.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. - PubMed
-
- Bechara A., Damasio A., Damasio H., Anderson S. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

