The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes
- PMID: 22721966
- PMCID: PMC3425423
- DOI: 10.2337/db11-1701
The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes
Abstract
In order to quantify the role of incretins in first- and second-phase insulin secretion (ISR) in type 2 diabetes mellitus (T2DM), a double-blind, randomized study with 12 T2DM subjects and 12 healthy subjects (HS) was conducted using the hyperglycemic clamp technique together with duodenal nutrition perfusion and intravenous infusion of the glucagon-like peptide 1 (GLP-1) receptor antagonist exendin(9-39). Intravenous glucose alone resulted in a significantly greater first- and second-phase ISR in HS compared with T2DM subjects. Duodenal nutrition perfusion augmented both first- and second-phase ISR but first-phase ISR more in T2DM subjects (approximately eight- vs. twofold). Glucose-related stimulation of ISR contributed only 20% to overall ISR. Infusion with exendin(9-39) significantly reduced first- and second-phase ISR in both HS and T2DM subjects. Thus, both GLP-1 and non-GLP-1 incretins contribute to the incretin effect. In conclusion, both phases of ISR are impaired in T2DM. In particular, the responsiveness to glucose in first-phase ISR is blunted. GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) secretions are unaltered. The absolute incretin effect is reduced in T2DM; its relative importance, however, appears to be increased, highlighting its role as an important amplifier of first-phase ISR in T2DM.
Trial registration: ClinicalTrials.gov NCT01449019.
Figures
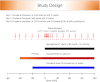
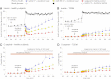
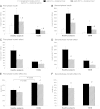

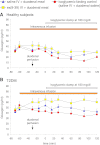
Comment in
-
Is endogenous GLP-1 the only important enhancer of glucose-induced insulin secretion in type 2 diabetes?Diabetes. 2012 Sep;61(9):2219-20. doi: 10.2337/db12-0649. Diabetes. 2012. PMID: 22923648 Free PMC article. No abstract available.
References
-
- Gerich JE. Contributions of insulin-resistance and insulin-secretory defects to the pathogenesis of type 2 diabetes mellitus. Mayo Clin Proc 2003;78:447–456 - PubMed
-
- Stumvoll M, Mitrakou A, Pimenta W, et al. Assessment of insulin secretion from the oral glucose tolerance test in white patients with type 2 diabetes. Diabetes Care 2000;23:1440–1441 - PubMed
-
- Pimenta W, Korytkowski M, Mitrakou A, et al. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 1995;273:1855–1861 - PubMed
-
- Woerle HJ, Szoke E, Meyer C, et al. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 2006;290:E67–E77 - PubMed
-
- Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 2008;294:E103–E109 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

