Large-scale prediction and testing of drug activity on side-effect targets
- PMID: 22722194
- PMCID: PMC3383642
- DOI: 10.1038/nature11159
Large-scale prediction and testing of drug activity on side-effect targets
Abstract
Discovering the unintended 'off-targets' that predict adverse drug reactions is daunting by empirical methods alone. Drugs can act on several protein targets, some of which can be unrelated by conventional molecular metrics, and hundreds of proteins have been implicated in side effects. Here we use a computational strategy to predict the activity of 656 marketed drugs on 73 unintended 'side-effect' targets. Approximately half of the predictions were confirmed, either from proprietary databases unknown to the method or by new experimental assays. Affinities for these new off-targets ranged from 1 nM to 30 μM. To explore relevance, we developed an association metric to prioritize those new off-targets that explained side effects better than any known target of a given drug, creating a drug-target-adverse drug reaction network. Among these new associations was the prediction that the abdominal pain side effect of the synthetic oestrogen chlorotrianisene was mediated through its newly discovered inhibition of the enzyme cyclooxygenase-1. The clinical relevance of this inhibition was borne out in whole human blood platelet aggregation assays. This approach may have wide application to de-risking toxicological liabilities in drug discovery.
Conflict of interest statement
Figures
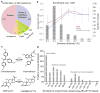

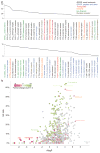
Comment in
-
Drug discovery: computer model predicts side effects.Nature. 2012 Jun 10;486(7403):326-7. doi: 10.1038/nature11198. Nature. 2012. PMID: 22722185 No abstract available.
References
-
- Giacomini KM, et al. When good drugs go bad. Nature. 2007;446:975–977. - PubMed
-
- Arrowsmith J. Trial watch: phase III and submission failures: 2007–2010. Nat Rev Drug Discov. 2011;10:87. - PubMed
-
- Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011;10:328–329. - PubMed
-
- Boyer S. The use of computer models in pharmaceutical safety evaluation. Altern Lab Anim. 2009;37:467–475. - PubMed
-
- Wang D, Wong D, Wang M, Cheng Y, Fitzgerald GA. Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr Opin Pharmacol. 2005;5:204–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

