Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors
- PMID: 22722203
- PMCID: PMC3383085
- DOI: 10.1038/nature11059
Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors
Abstract
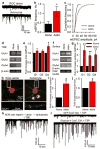
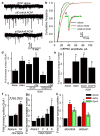
In the developing central nervous system (CNS), the control of synapse number and function is critical to the formation of neural circuits. We previously demonstrated that astrocyte-secreted factors powerfully induce the formation of functional excitatory synapses between CNS neurons. Astrocyte-secreted thrombospondins induce the formation of structural synapses, but these synapses are postsynaptically silent. Here we use biochemical fractionation of astrocyte-conditioned medium to identify glypican 4 (Gpc4) and glypican 6 (Gpc6) as astrocyte-secreted signals sufficient to induce functional synapses between purified retinal ganglion cell neurons, and show that depletion of these molecules from astrocyte-conditioned medium significantly reduces its ability to induce postsynaptic activity. Application of Gpc4 to purified neurons is sufficient to increase the frequency and amplitude of glutamatergic synaptic events. This is achieved by increasing the surface level and clustering, but not overall cellular protein level, of the GluA1 subunit of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) glutamate receptor (AMPAR). Gpc4 and Gpc6 are expressed by astrocytes in vivo in the developing CNS, with Gpc4 expression enriched in the hippocampus and Gpc6 enriched in the cerebellum. Finally, we demonstrate that Gpc4-deficient mice have defective synapse formation, with decreased amplitude of excitatory synaptic currents in the developing hippocampus and reduced recruitment of AMPARs to synapses. These data identify glypicans as a family of novel astrocyte-derived molecules that are necessary and sufficient to promote glutamate receptor clustering and receptivity and to induce the formation of postsynaptically functioning CNS synapses.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

