Fluorescence-guided surgical sampling of glioblastoma identifies phenotypically distinct tumour-initiating cell populations in the tumour mass and margin
- PMID: 22722315
- PMCID: PMC3405212
- DOI: 10.1038/bjc.2012.271
Fluorescence-guided surgical sampling of glioblastoma identifies phenotypically distinct tumour-initiating cell populations in the tumour mass and margin
Abstract
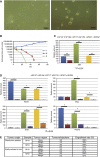
Background: Acquiring clinically annotated, spatially stratified tissue samples from human glioblastoma (GBM) is compromised by haemorrhage, brain shift and subjective identification of 'normal' brain. We tested the use of 5-aminolevulinic acid (5-ALA) fluorescence to objective tissue sampling and to derive tumour-initiating cells (TICs) from mass and margin.
Methods: The 5-ALA was administered to 30 GBM patients. Samples were taken from the non-fluorescent necrotic core, fluorescent tumour mass and non-fluorescent margin. We compared the efficiency of isolating TICs from these areas in 5-ALA versus control patients. HRMAS (1)H NMR was used to reveal metabolic alterations due to 5-ALA. We then characterised TICs for self-renewal in vitro and tumorigenicity in vivo.
Results: The derivation of TICs was not compromised by 5-ALA and the metabolic profile was similar between tumours from 5-ALA patients and controls. The TICs from the fluorescent mass were self-renewing in vitro and tumour-forming in vivo, whereas TICs from non-fluorescent margin did not self-renew in vitro but did form tumours in vivo.
Conclusion: Our data show that 5-ALA does not compromise the derivation of TICs. It also reveals that the margin contains TICs, which are phenotypically different from those isolated from the corresponding mass.
© 2012 Cancer Research UK
Figures

References
-
- Barrett LE, Granot Z, Coker C, Iavarone A, Hambardzumyan D, Holland EC, Nam HS, Benezra R (2012) Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell 21(1): 11–24 - PubMed
-
- Fael Al-Mayhani TM, Ball SL, Zhao JW, Fawcett J, Ichimura K, Collins PV, Watts C (2009) An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. J Neurosci Methods 176(2): 192–199 - PubMed
-
- Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG (2010) NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28(1): 5–16 - PMC - PubMed
-
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131(15): 3805–3819 - PubMed
-
- Gaiano N, Fishell G (2002) The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci 25: 471–490 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

