A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder
- PMID: 22722512
- PMCID: PMC3438886
- DOI: 10.1097/JCP.0b013e31825d70d6
A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder
Abstract
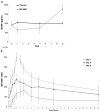
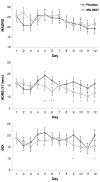
Converging lines of evidence suggest that the glutamatergic system may play an increasingly important role in the development of novel therapeutics for major depressive disorder (MDD), particularly agents associated with rapid antidepressant effects. Diverse glutamatergic modulators targeting N-methyl-D-aspartate receptors have shown efficacy in MDD, but their associated psychotomimetic effects presently preclude their use in larger samples. This small, randomized, double-blind, placebo-controlled, crossover pilot study evaluated the potential antidepressant efficacy and tolerability of an oral formulation of the selective N-methyl-D-aspartate NR2B antagonist MK-0657 in patients with treatment-resistant MDD (TRD). The TRD subjects underwent a 1-week drug-free period and were subsequently randomized to receive either MK-0657 monotherapy (4-8 mg/d) or placebo for 12 days. Because of recruitment challenges and the discontinuation of the compound's development by the manufacturer, only 5 of the planned 21 patients completed both periods of the crossover administration of MK-0657 and placebo. Significant antidepressant effects were observed as early as day 5 in patients receiving MK-0657 compared with those receiving placebo, as assessed by the Hamilton Depression Rating Scale and Beck Depression Inventory; however, no improvement was noted when symptoms were assessed with the Montgomery-Asberg Depression Rating Scale, the primary efficacy measure. No serious or dissociative adverse effects were observed in patients receiving this oral formulation of MK-0657. Despite the small sample size, this pilot study suggests that an oral formulation of the NR2B antagonist MK-0657 may have antidepressant properties in TRD patients. Further studies with larger sample sizes are necessary to confirm these preliminary findings.
Trial registration: ClinicalTrials.gov NCT00472576.
Figures
References
-
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. - PubMed
-
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. - PubMed
-
- Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

