Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts
- PMID: 22722650
- PMCID: PMC4594775
- DOI: 10.1159/000337654
Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts
Abstract
Background: Diagnosis and immunotherapy of house-dust mite (HDM) allergy is still based on natural allergen extracts. The aim of this study was to analyze commercially available Dermatophagoides pteronyssinus extracts from different manufacturers regarding allergen composition and content and whether variations may affect their allergenic activity.
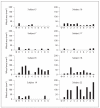
Methods: Antibodies specific for several D. pteronyssinus allergens (Der p 1, 2, 5, 7, 10 and 21) were used to analyze extracts from 10 different manufacturers by immunoblotting. Sandwich ELISAs were used to quantify Der p 1 and Der p 2 in the extracts. Mite-allergic patients (n = 45) were skin-tested with the extracts and tested for immunoglobulin E (IgE) reactivity to a panel of 10 mite allergens (Der p 1, 2, 4, 5, 7, 8, 10, 14, 20 and 21) by dot blot.
Results: Only Der p 1 and Der p 2 were detected in all extracts but their concentrations and ratios showed high variability (Der p 1: 6.0-40.8 µg ml(-1); Der p 2: 1.7-45.0 µg ml(-1)). At least 1 out of 4 allergens (i.e. Der p 5, 7, 10 and 21) was not detected in 8 of the studied extracts. Mite-allergic subjects showed different IgE reactivity profiles to the individual mite allergens, the extracts showed different allergenic activity in skin-prick tests and false-negative results.
Conclusions: Commercially available D. pteronyssinus extracts lack important allergens, show great variability regarding allergen composition and content and some gave false-negative diagnostic test results in certain patients.
Copyright © 2012 S. Karger AG, Basel.
Figures


Comment in
-
Quality control of house dust mite extracts for allergen immunotherapy.Int Arch Allergy Immunol. 2013;161(3):285-6. doi: 10.1159/000347045. Epub 2013 Mar 15. Int Arch Allergy Immunol. 2013. PMID: 23548609 No abstract available.
-
Allergen content and in vivo allergenic activity of house dust mite extracts.Int Arch Allergy Immunol. 2013;161(3):287-8. doi: 10.1159/000347047. Epub 2013 Mar 15. Int Arch Allergy Immunol. 2013. PMID: 23548663 Free PMC article. No abstract available.
References
-
- Kern RA. Dust sensitization in bronchial asthma. Med Clin North Am. 1921;5:751–758.
-
- Voorhorst R, Spieksma-Boezman MIA, Spieksma FTHM. Is a mite (Dermatophagoides spp.) the producer of the house dust allergen? Allerg Asthma. 1964;10:329–334. - PubMed
-
- Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. - PubMed
-
- Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007;14:943–953. - PubMed
-
- Weghofer M, Dall’Antonia Y, Grote M, Stöcklinger A, Kneidinger M, Balic N, Krauth MT, Fernandez-Caldas E, Thomas WR, van Hage M, Vieths S, Spitzauer S, Horak F, Svergun DI, Konarev PV, Valent P, Thalhamer J, Keller W, Valenta R, Vrtala S. Characterization of Der p 21, a new important allergen derived from the gut of house dust mites. Allergy. 2008;63:758–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

