Novel mutations target distinct subgroups of medulloblastoma
- PMID: 22722829
- PMCID: PMC3412905
- DOI: 10.1038/nature11213
Novel mutations target distinct subgroups of medulloblastoma
Abstract
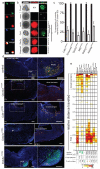
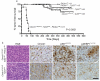
Medulloblastoma is a malignant childhood brain tumour comprising four discrete subgroups. Here, to identify mutations that drive medulloblastoma, we sequenced the entire genomes of 37 tumours and matched normal blood. One-hundred and thirty-six genes harbouring somatic mutations in this discovery set were sequenced in an additional 56 medulloblastomas. Recurrent mutations were detected in 41 genes not yet implicated in medulloblastoma; several target distinct components of the epigenetic machinery in different disease subgroups, such as regulators of H3K27 and H3K4 trimethylation in subgroups 3 and 4 (for example, KDM6A and ZMYM3), and CTNNB1-associated chromatin re-modellers in WNT-subgroup tumours (for example, SMARCA4 and CREBBP). Modelling of mutations in mouse lower rhombic lip progenitors that generate WNT-subgroup tumours identified genes that maintain this cell lineage (DDX3X), as well as mutated genes that initiate (CDH1) or cooperate (PIK3CA) in tumorigenesis. These data provide important new insights into the pathogenesis of medulloblastoma subgroups and highlight targets for therapeutic development.
Figures


References
-
- CBTRUS . Statistical Report: Primary Brain Tumors in the United States, 1995-1999. Central Brain Tumor Registry of the United States; Hinsdale, IL: 2006. 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

