PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells
- PMID: 22722857
- PMCID: PMC3387339
- DOI: 10.1038/nature11132
PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells
Abstract
Obesity and type-2 diabetes have increased markedly over the past few decades, in parallel. One of the major links between these two disorders is chronic, low-grade inflammation. Prolonged nutrient excess promotes the accumulation and activation of leukocytes in visceral adipose tissue (VAT) and ultimately other tissues, leading to metabolic abnormalities such as insulin resistance, type-2 diabetes and fatty-liver disease. Although invasion of VAT by pro-inflammatory macrophages is considered to be a key event driving adipose-tissue inflammation and insulin resistance, little is known about the roles of other immune system cell types in these processes. A unique population of VAT-resident regulatory T (Treg) cells was recently implicated in control of the inflammatory state of adipose tissue and, thereby, insulin sensitivity. Here we identify peroxisome proliferator-activated receptor (PPAR)-γ, the 'master regulator' of adipocyte differentiation, as a crucial molecular orchestrator of VAT Treg cell accumulation, phenotype and function. Unexpectedly, PPAR-γ expression by VAT Treg cells was necessary for complete restoration of insulin sensitivity in obese mice by the thiazolidinedione drug pioglitazone. These findings suggest a previously unknown cellular mechanism for this important class of thiazolidinedione drugs, and provide proof-of-principle that discrete populations of Treg cells with unique functions can be precisely targeted to therapeutic ends.
Conflict of interest statement
Figures
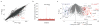
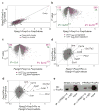
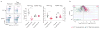

Comment in
-
Regulatory T cells expressing PPAR-γ control inflammation in obesity.Cell Metab. 2012 Jul 3;16(1):4-6. doi: 10.1016/j.cmet.2012.06.007. Cell Metab. 2012. PMID: 22768833
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

