Conformational changes relevant to channel activity and folding within the first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator
- PMID: 22722932
- PMCID: PMC3436552
- DOI: 10.1074/jbc.M112.371138
Conformational changes relevant to channel activity and folding within the first nucleotide binding domain of the cystic fibrosis transmembrane conductance regulator
Abstract
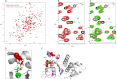
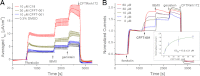
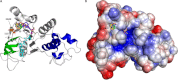
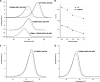
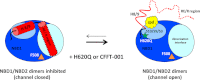
Deletion of Phe-508 (F508del) in the first nucleotide binding domain (NBD1) of the cystic fibrosis transmembrane conductance regulator (CFTR) leads to defects in folding and channel gating. NMR data on human F508del NBD1 indicate that an H620Q mutant, shown to increase channel open probability, and the dual corrector/potentiator CFFT-001 similarly disrupt interactions between β-strands S3, S9, and S10 and the C-terminal helices H8 and H9, shifting a preexisting conformational equilibrium from helix to coil. CFFT-001 appears to interact with β-strands S3/S9/S10, consistent with docking simulations. Decreases in T(m) from differential scanning calorimetry with H620Q or CFFT-001 suggest direct compound binding to a less thermostable state of NBD1. We hypothesize that, in full-length CFTR, shifting the conformational equilibrium to reduce H8/H9 interactions with the uniquely conserved strands S9/S10 facilitates release of the regulatory region from the NBD dimerization interface to promote dimerization and thereby increase channel open probability. These studies enabled by our NMR assignments for F508del NBD1 provide a window into the conformational fluctuations within CFTR that may regulate function and contribute to folding energetics.
Figures




References
-
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. (1989) Identification of the cystic fibrosis gene: Genetic analysis. Science 245, 1073–1080 - PubMed
-
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. (1989) Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 245, 1059–1065 - PubMed
-
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. (1989) Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245, 1066–1073 - PubMed
-
- Lewis H. A., Buchanan S. G., Burley S. K., Conners K., Dickey M., Dorwart M., Fowler R., Gao X., Guggino W. B., Hendrickson W. A., Hunt J. F., Kearins M. C., Lorimer D., Maloney P. C., Post K. W., Rajashankar K. R., Rutter M. E., Sauder J. M., Shriver S., Thibodeau P. H., Thomas P. J., Zhang M., Zhao X., Emtage S. (2004) Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 23, 282–293 - PMC - PubMed
-
- Ostedgaard L. S., Baldursson O., Welsh M. J. (2001) Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by its R domain. J. Biol. Chem. 276, 7689–7692 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

