Control of pollen-mediated gene flow in transgenic trees
- PMID: 22723085
- PMCID: PMC3425181
- DOI: 10.1104/pp.112.197228
Control of pollen-mediated gene flow in transgenic trees
Abstract
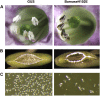
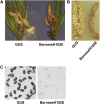
Pollen elimination provides an effective containment method to reduce direct gene flow from transgenic trees to their wild relatives. Until now, only limited success has been achieved in controlling pollen production in trees. A pine (Pinus radiata) male cone-specific promoter, PrMC2, was used to drive modified barnase coding sequences (barnaseH102E, barnaseK27A, and barnaseE73G) in order to determine their effectiveness in pollen ablation. The expression cassette PrMC2-barnaseH102E was found to efficiently ablate pollen in tobacco (Nicotiana tabacum), pine, and Eucalyptus (spp.). Large-scale and multiple-year field tests demonstrated that complete prevention of pollen production was achieved in greater than 95% of independently transformed lines of pine and Eucalyptus (spp.) that contained the PrMC2-barnaseH102E expression cassette. A complete pollen control phenotype was achieved in transgenic lines and expressed stably over multiple years, multiple test locations, and when the PrMC2-barnaseH102E cassette was flanked by different genes. The PrMC2-barnaseH102E transgenic pine and Eucalyptus (spp.) trees grew similarly to control trees in all observed attributes except the pollenless phenotype. The ability to achieve the complete control of pollen production in field-grown trees is likely the result of a unique combination of three factors: the male cone/anther specificity of the PrMC2 promoter, the reduced RNase activity of barnaseH102E, and unique features associated with a polyploid tapetum. The field performance of the PrMC2-barnaseH102E in representative angiosperm and gymnosperm trees indicates that this gene can be used to mitigate pollen-mediated gene flow associated with large-scale deployment of transgenic trees.
Figures





References
-
- Axe DD, Foster NW, Fersht AR. (1998) A search for single substitutions that eliminate enzymatic function in a bacterial ribonuclease. Biochemistry 37: 7157–7166 - PubMed
-
- Bramlett DL, Bridgwater FE. (1989) Pollen development classification system for loblolly pine. In Proceedings of the 20th Southern Forest Tree Improvement Conference. National Reforestation, Nurseries, and Genetic Resources (RNGR) Program, USDA Forest Service, Washington, DC, pp 116–121
-
- Bramlett DL, Williams CG, Burris LC. (1995) Surrogate pollen induction shortens the breeding cycle in loblolly pine. Tree Physiol 15: 531–535 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

