Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice
- PMID: 22723263
- PMCID: PMC3423140
- DOI: 10.1152/ajpgi.00120.2012
Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice
Abstract
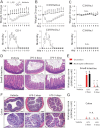
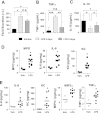
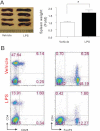
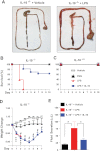
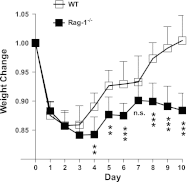
Frequency of gram-negative bacteria is markedly enhanced in inflamed gut, leading to augmented LPS in the intestine. Although LPS in the intestine is considered harmless and, rather, provides protective effects against epithelial injury, it has been suggested that LPS causes intestinal inflammation, such as necrotizing enterocolitis. Therefore, direct effects of LPS in the intestine remain to be studied. In this study, we examine the effect of LPS in the colon of mice instilled with LPS by rectal enema. We found that augmented LPS on the luminal side of the colon elicited inflammation in the small intestine remotely, not in the colon; this inflammation was characterized by body weight loss, increased fluid secretion, enhanced inflammatory cytokine production, and epithelial damage. In contrast to the inflamed small intestine induced by colonic LPS, the colonic epithelium did not exhibit histological tissue damage or inflammatory lesions, although intracolonic LPS treatment elicited inflammatory cytokine gene expression in the colon tissues. Moreover, we found that intracolonic LPS treatment substantially decreased the frequency of immune-suppressive regulatory T cells (CD4(+)/CD25(+) and CD4(+)/Foxp3(+)). We were intrigued to find that LPS-promoted intestinal inflammation is exacerbated in immune modulator-impaired IL-10(-/-) and Rag-1(-/-) mice. In conclusion, our results provide evidence that elevated LPS in the colon is able to cause intestinal inflammation and, therefore, suggest a physiological explanation for the importance of maintaining the balance between gram-negative and gram-positive bacteria in the intestine to maintain homeostasis in the gut.
Figures
References
-
- Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem 277: 20431–20437, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

