Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury
- PMID: 22723264
- PMCID: PMC3468550
- DOI: 10.1152/ajpgi.00431.2011
Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury
Abstract
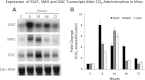
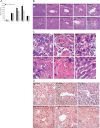
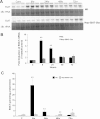
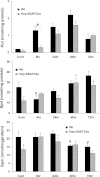
Activation of spermine/spermidine-N(1)-acetyltransferase (SSAT) leads to DNA damage and growth arrest in mammalian cells, and its ablation reduces the severity of ischemic and endotoxic injuries. Here we have examined the role of SSAT in the pathogenesis of toxic liver injury caused by carbon tetrachloride (CCl(4)). The expression and activity of SSAT increase in the liver subsequent to CCl(4) administration. Furthermore, the early liver injury after CCl(4) treatment was significantly attenuated in hepatocyte-specific SSAT knockout mice (Hep-SSAT-Cko) compared with wild-type (WT) mice as determined by the reduced serum alanine aminotransferase levels, decreased hepatic lipid peroxidation, and less severe liver damage. Cytochrome P450 2e1 levels remained comparable in both genotypes, suggesting that SSAT deficiency does not affect the metabolism of CCl(4). Hepatocyte-specific deficiency of SSAT also modulated the induction of cytokines involved in inflammation and repair as well as leukocyte infiltration. In addition, Noxa and activated caspase 3 levels were elevated in the livers of WT compared with Hep-SSAT-Cko mice. Interestingly, the onset of cell proliferation was significantly more robust in the WT compared with Hep-SSAT Cko mice. The inhibition of polyamine oxidases protected the animals against CCl(4)-induced liver injury. Our studies suggest that while the abrogation of polyamine back conversion or inhibition of polyamine oxidation attenuate the early injury, they may delay the onset of hepatic regeneration.
Figures








References
-
- Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005 - PubMed
-
- Baskaya MK, Rao AM, Dogan A, Donaldson D, Gellin G, Dempsey RJ. Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res 744: 302–308, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

