Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction
- PMID: 22723297
- PMCID: PMC4219578
- DOI: 10.1161/CIRCRESAHA.112.274530
Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction
Abstract
Rationale: Transverse tubules (TTs) couple electric surface signals to remote intracellular Ca(2+) release units (CRUs). Diffraction-limited imaging studies have proposed loss of TT components as disease mechanism in heart failure (HF).
Objectives: Objectives were to develop quantitative super-resolution strategies for live-cell imaging of TT membranes in intact cardiomyocytes and to show that TT structures are progressively remodeled during HF development, causing early CRU dysfunction.
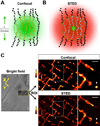
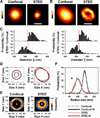
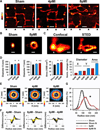
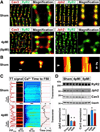
Methods and results: Using stimulated emission depletion (STED) microscopy, we characterized individual TTs with nanometric resolution as direct readout of local membrane morphology 4 and 8 weeks after myocardial infarction (4pMI and 8pMI). Both individual and network TT properties were investigated by quantitative image analysis. The mean area of TT cross sections increased progressively from 4pMI to 8pMI. Unexpectedly, intact TT networks showed differential changes. Longitudinal and oblique TTs were significantly increased at 4pMI, whereas transversal components appeared decreased. Expression of TT-associated proteins junctophilin-2 and caveolin-3 was significantly changed, correlating with network component remodeling. Computational modeling of spatial changes in HF through heterogeneous TT reorganization and RyR2 orphaning (5000 of 20 000 CRUs) uncovered a local mechanism of delayed subcellular Ca(2+) release and action potential prolongation.
Conclusions: This study introduces STED nanoscopy for live mapping of TT membrane structures. During early HF development, the local TT morphology and associated proteins were significantly altered, leading to differential network remodeling and Ca(2+) release dyssynchrony. Our data suggest that TT remodeling during HF development involves proliferative membrane changes, early excitation-contraction uncoupling, and network fracturing.
Figures

References
-
- Endo M. Entry of a dye into the sarcotubular system of muscle. Nature. 1964;202:1115–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

