Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement
- PMID: 22723355
- PMCID: PMC3390832
- DOI: 10.1073/pnas.1208698109
Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement
Abstract
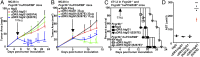
By virtue of their ability to induce apoptosis and regulate growth, differentiation, and cytokine responses, the tumor necrosis factor receptor (TNFR) superfamily members have emerged as attractive targets for anticancer therapeutics. Agonistic antibodies to apoptosis-inducing TNFRs, such as death receptor 5 (DR5), although displaying impressive activities against a variety of tumors in preclinical models, appear to be less active in clinical trials. We report that the in vivo apoptotic and antitumor activities of these antibodies have an absolute requirement for the coengagement of an inhibitory Fcγ receptor, FcγRIIB. Anti-DR5 antibodies of the type currently in clinical trials have weak FcγRIIB binding and thus are compromised in their proapoptotic and antitumor activities in both colon and breast carcinoma models. Enhancing FcγRIIB engagement increases apoptotic and antitumor potency. Our results demonstrate that Fc domain interactions are critical to the therapeutic activity of anti-DR5 antibodies and, together with previous reports on agonistic anti-CD40 antibodies, establish a common requirement for FcγRIIB coengagement for optimal biological effects of agonistic anti-TNFR antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



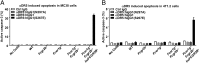

References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Grewal IS. Therapeutic Targets of the TNF Superfamily. New York, NY: Springer; 2009. pp. 1–220.
-
- Daniel PT, Wieder T, Sturm I, Schulze-Osthoff K. The kiss of death: Promises and failures of death receptors and ligands in cancer therapy. Leukemia. 2001;15:1022–1032. - PubMed
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. - PubMed
-
- Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: The opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

