Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS
- PMID: 22723552
- PMCID: PMC3418722
- DOI: 10.1182/blood-2012-01-404699
Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS
Abstract
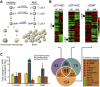
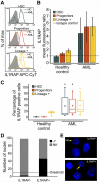
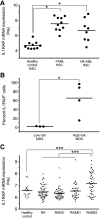
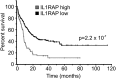
Cellular and interpatient heterogeneity and the involvement of different stem and progenitor compartments in leukemogenesis are challenges for the identification of common pathways contributing to the initiation and maintenance of acute myeloid leukemia (AML). Here we used a strategy of parallel transcriptional analysis of phenotypic long-term hematopoietic stem cells (HSCs), short-term HSCs, and granulocyte-monocyte progenitors from individuals with high-risk (-7/7q-) AML and compared them with the corresponding cell populations from healthy controls. This analysis revealed dysregulated expression of 11 genes, including IL-1 receptor accessory protein (IL1RAP), in all leukemic stem and progenitor cell compartments. IL1RAP protein was found to be overexpressed on the surface of HSCs of AML patients, and marked cells with the -7/7q- anomaly. IL1RAP was also overexpressed on HSCs of patients with normal karyotype AML and high-risk myelodysplastic syndrome, suggesting a pervasive role in different disease subtypes. High IL1RAP expression was independently associated with poor overall survival in 3 independent cohorts of AML patients (P = 2.2 × 10(-7)). Knockdown of IL1RAP decreased clonogenicity and increased cell death of AML cells. Our study identified genes dysregulated in stem and progenitor cells in -7/7q- AML, and suggests that IL1RAP may be a promising therapeutic and prognostic target in AML and high-risk myelodysplastic syndrome.
Figures

References
-
- Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

