NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension
- PMID: 22723696
- PMCID: PMC3390258
- DOI: 10.1523/JNEUROSCI.1346-12.2012
NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension
Abstract
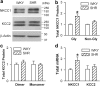
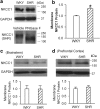
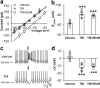
Hypertension is a major risk factor for coronary artery disease, stroke, and kidney failure. However, the etiology of hypertension in most patients is poorly understood. Increased sympathetic drive emanating from the hypothalamic paraventricular nucleus (PVN) plays a major role in the development of hypertension. Na(+)-K(+)-2Cl(-) cotransporter-1 (NKCC1) in the brain is critically involved in maintaining chloride homeostasis and in neuronal responses mediated by GABA(A) receptors. Here we present novel evidence that the GABA reversal potential (E(GABA)) of PVN presympathetic neurons undergoes a depolarizing shift that diminishes GABA inhibition in spontaneously hypertensive rats (SHRs). Inhibition of NKCC1, but not KCC2, normalizes E(GABA) and restores GABA inhibition of PVN neurons in SHRs. The mRNA and protein levels of NKCC1, but not KCC2, in the PVN are significantly increased in SHRs, and the NKCC1 proteins on the plasma membrane are highly glycosylated. Inhibiting NKCC1 N-glycosylation restores E(GABA) and GABAergic inhibition of PVN presympathetic neurons in SHRs. Furthermore, NKCC1 inhibition significantly reduces the sympathetic vasomotor tone and augments the sympathoinhibitory responses to GABA(A) receptor activation in the PVN in SHRs. These findings suggest that increased NKCC1 activity and glycosylation disrupt chloride homeostasis and impair synaptic inhibition in the PVN to augment the sympathetic drive in hypertension. This information greatly improves our understanding of the pathogenesis of hypertension and helps to design better treatment strategies for neurogenic hypertension.
Figures





References
-
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. - PubMed
-
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. - PubMed
-
- Ciriello J. Forebrain mechanisms in neurogenic hypertension. Can J Physiol Pharmacol. 1987;65:1580–1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
