Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans
- PMID: 22723742
- PMCID: PMC3378601
- DOI: 10.1371/journal.pbio.1001346
Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans
Abstract
How small heat shock proteins (sHsps) might empower proteostasis networks to control beneficial prions or disassemble pathological amyloid is unknown. Here, we establish that yeast sHsps, Hsp26 and Hsp42, inhibit prionogenesis by the [PSI+] prion protein, Sup35, via distinct and synergistic mechanisms. Hsp42 prevents conformational rearrangements within molten oligomers that enable de novo prionogenesis and collaborates with Hsp70 to attenuate self-templating. By contrast, Hsp26 inhibits self-templating upon binding assembled prions. sHsp binding destabilizes Sup35 prions and promotes their disaggregation by Hsp104, Hsp70, and Hsp40. In yeast, Hsp26 or Hsp42 overexpression prevents [PSI+] induction, cures [PSI+], and potentiates [PSI+]-curing by Hsp104 overexpression. In vitro, sHsps enhance Hsp104-catalyzed disaggregation of pathological amyloid forms of α-synuclein and polyglutamine. Unexpectedly, in the absence of Hsp104, sHsps promote an unprecedented, gradual depolymerization of Sup35 prions by Hsp110, Hsp70, and Hsp40. This unanticipated amyloid-depolymerase activity is conserved from yeast to humans, which lack Hsp104 orthologues. A human sHsp, HspB5, stimulates depolymerization of α-synuclein amyloid by human Hsp110, Hsp70, and Hsp40. Thus, we elucidate a heretofore-unrecognized human amyloid-depolymerase system that could have applications in various neurodegenerative disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



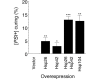
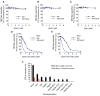


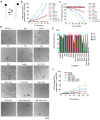

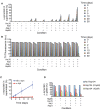
References
-
- Chiti F, Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Fowler D. M, Koulov A. V, Alory-Jost C, Marks M. S, Balch W. E, et al. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

