Imatinib enhances functional outcome after spinal cord injury
- PMID: 22723886
- PMCID: PMC3378614
- DOI: 10.1371/journal.pone.0038760
Imatinib enhances functional outcome after spinal cord injury
Abstract
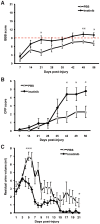
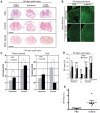
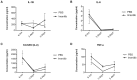
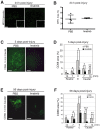
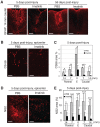
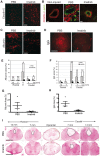
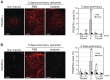
We investigated whether imatinib (Gleevec®, Novartis), a tyrosine kinase inhibitor, could improve functional outcome in experimental spinal cord injury. Rats subjected to contusion spinal cord injury were treated orally with imatinib for 5 days beginning 30 minutes after injury. We found that imatinib significantly enhanced blood-spinal cord-barrier integrity, hindlimb locomotor function, sensorimotor integration, and bladder function, as well as attenuated astrogliosis and deposition of chondroitin sulfate proteoglycans, and increased tissue preservation. These improvements were associated with enhanced vascular integrity and reduced inflammation. Our results show that imatinib improves recovery in spinal cord injury by preserving axons and other spinal cord tissue components. The rapid time course of these beneficial effects suggests that the effects of imatinib are neuroprotective rather than neurorestorative. The positive effects on experimental spinal cord injury, obtained by oral delivery of a clinically used drug, makes imatinib an interesting candidate drug for clinical trials in spinal cord injury.
Conflict of interest statement
Figures
References
-
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. - PubMed
-
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. - PubMed
-
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. - PubMed
-
- Neumann H, Wekerle H. Neuronal control of the immune response in the central nervous system: linking brain immunity to neurodegeneration. J Neuropathol Exp Neurol. 1998;57:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

