Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells
- PMID: 22723910
- PMCID: PMC3379171
- DOI: 10.1371/journal.pone.0038941
Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells
Abstract
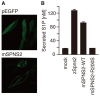
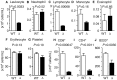
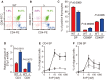
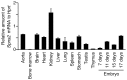
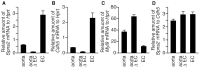
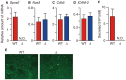
Sphingosine-1-phosphate (S1P), a sphingolipid metabolite that is produced inside the cells, regulates a variety of physiological and pathological responses via S1P receptors (S1P1-5). Signal transduction between cells consists of three steps; the synthesis of signaling molecules, their export to the extracellular space and their recognition by receptors. An S1P concentration gradient is essential for the migration of various cell types that express S1P receptors, such as lymphocytes, pre-osteoclasts, cancer cells and endothelial cells. To maintain this concentration gradient, plasma S1P concentration must be at a higher level. However, little is known about the molecular mechanism by which S1P is supplied to extracellular environments such as blood plasma. Here, we show that SPNS2 functions as an S1P transporter in vascular endothelial cells but not in erythrocytes and platelets. Moreover, the plasma S1P concentration of SPNS2-deficient mice was reduced to approximately 60% of wild-type, and SPNS2-deficient mice were lymphopenic. Our results demonstrate that SPNS2 is the first physiological S1P transporter in mammals and is a key determinant of lymphocyte egress from the thymus.
Conflict of interest statement
Figures





References
-
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annual Review of Biochemistry. 2009;78:743–768. - PubMed
-
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. - PubMed
-
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature Immunology. 2007;8:1295–1301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

