Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study
- PMID: 22723916
- PMCID: PMC3377625
- DOI: 10.1371/journal.pone.0038998
Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study
Abstract
Background: Kawasaki disease is recognized as the most common cause of acquired heart disease in children in the developed world. Clinical, epidemiologic, and pathologic evidence supports an infectious agent, likely entering through the lung. Pathologic studies proposing an acute coronary arteritis followed by healing fail to account for the complex vasculopathy and clinical course.
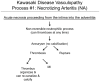
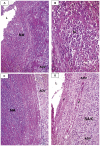
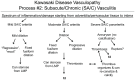
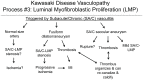
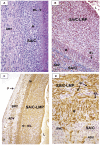
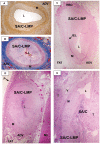
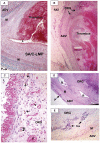
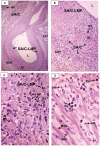
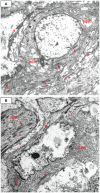
Methodology/principal findings: Specimens from 32 autopsies, 8 cardiac transplants, and an excised coronary aneurysm were studied by light (n=41) and transmission electron microscopy (n=7). Three characteristic vasculopathic processes were identified in coronary (CA) and non-coronary arteries: acute self-limited necrotizing arteritis (NA), subacute/chronic (SA/C) vasculitis, and luminal myofibroblastic proliferation (LMP). NA is a synchronous neutrophilic process of the endothelium, beginning and ending within the first two weeks of fever onset, and progressively destroying the wall into the adventitia causing saccular aneurysms, which can thrombose or rupture. SA/C vasculitis is an asynchronous process that can commence within the first two weeks onward, starting in the adventitia/perivascular tissue and variably inflaming/damaging the wall during progression to the lumen. Besides fusiform and saccular aneurysms that can thrombose, SA/C vasculitis likely causes the transition of medial and adventitial smooth muscle cells (SMC) into classic myofibroblasts, which combined with their matrix products and inflammation create progressive stenosing luminal lesions (SA/C-LMP). Remote LMP apparently results from circulating factors. Veins, pulmonary arteries, and aorta can develop subclinical SA/C vasculitis and SA/C-LMP, but not NA. The earliest death (day 10) had both CA SA/C vasculitis and SA/C-LMP, and an "eosinophilic-type" myocarditis.
Conclusions/significance: NA is the only self-limiting process of the three, is responsible for the earliest morbidity/mortality, and is consistent with acute viral infection. SA/C vasculitis can begin as early as NA, but can occur/persist for months to years; LMP causes progressive arterial stenosis and thrombosis and is composed of unique SMC-derived pathologic myofibroblasts.
Conflict of interest statement
Figures






References
-
- Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967;16:178–222. - PubMed
-
- Landing BH, Larson EJ. Are infantile periarteritis nodosa with coronary artery involvement and fatal mucocutaneous lymph node syndrome the same? Comparison of 20 patients from North America with patients from Hawaii and Japan. Pediatrics. 1977;59:651–662. - PubMed
-
- Tanaka N, Sekimoto K, Naoe S. Kawasaki disease. Relationship with infantile periarteritis nodosa. Arch Pathol Lab Med. 1976;100:81–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

