Intracellular invasion of Orientia tsutsugamushi activates inflammasome in asc-dependent manner
- PMID: 22723924
- PMCID: PMC3377614
- DOI: 10.1371/journal.pone.0039042
Intracellular invasion of Orientia tsutsugamushi activates inflammasome in asc-dependent manner
Abstract
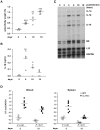
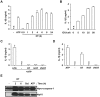
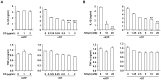
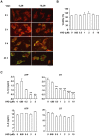
Orientia tsutsugamushi, a causative agent of scrub typhus, is an obligate intracellular bacterium, which escapes from the endo/phagosome and replicates in the host cytoplasm. O. tsutsugamushi infection induces production of pro-inflammatory mediators including interleukin-1β (IL-1β), which is secreted mainly from macrophages upon cytosolic stimuli by activating cysteine protease caspase-1 within a complex called the inflammasome, and is a key player in initiating and maintaining the inflammatory response. However, the mechanism for IL-1β maturation upon O. tsutsugamushi infection has not been identified. In this study, we show that IL-1 receptor signaling is required for efficient host protection from O. tsutsugamushi infection. Live Orientia, but not heat- or UV-inactivated Orientia, activates the inflammasome through active bacterial uptake and endo/phagosomal maturation. Furthermore, Orientia-stimulated secretion of IL-1β and activation of caspase-1 are ASC- and caspase-1- dependent since IL-1β production was impaired in Asc- and caspase-1-deficient macrophages but not in Nlrp3-, Nlrc4- and Aim2-deficient macrophages. Therefore, live O. tsutsugamushi triggers ASC inflammasome activation leading to IL-1β production, which is a critical innate immune response for effective host defense.
Conflict of interest statement
Figures
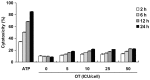
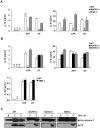
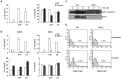
Similar articles
-
Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection.J Immunol. 2013 Apr 1;190(7):3629-38. doi: 10.4049/jimmunol.1202817. Epub 2013 Mar 4. J Immunol. 2013. PMID: 23460746
-
A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages.PLoS Pathog. 2011 Apr;7(4):e1002026. doi: 10.1371/journal.ppat.1002026. Epub 2011 Apr 21. PLoS Pathog. 2011. PMID: 21533069 Free PMC article.
-
Caspase-1/ASC inflammasome-mediated activation of IL-1β-ROS-NF-κB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3-/- macrophages.PLoS One. 2014 Nov 5;9(11):e111539. doi: 10.1371/journal.pone.0111539. eCollection 2014. PLoS One. 2014. PMID: 25372293 Free PMC article.
-
Structural mechanisms in NLR inflammasome signaling.Curr Opin Struct Biol. 2014 Dec;29:17-25. doi: 10.1016/j.sbi.2014.08.011. Epub 2014 Sep 15. Curr Opin Struct Biol. 2014. PMID: 25201319 Free PMC article. Review.
-
Research progress on the NLRP3 inflammasome and its role in the central nervous system.Neurosci Bull. 2013 Dec;29(6):779-87. doi: 10.1007/s12264-013-1328-9. Epub 2013 Mar 20. Neurosci Bull. 2013. PMID: 23512739 Free PMC article. Review.
Cited by
-
Scrub Typhus Pathogenesis: Innate Immune Response and Lung Injury During Orientia tsutsugamushi Infection.Front Microbiol. 2019 Sep 6;10:2065. doi: 10.3389/fmicb.2019.02065. eCollection 2019. Front Microbiol. 2019. PMID: 31555249 Free PMC article. Review.
-
Orientia tsutsugamushi selectively stimulates the C-type lectin receptor Mincle and type 1-skewed proinflammatory immune responses.PLoS Pathog. 2021 Jul 28;17(7):e1009782. doi: 10.1371/journal.ppat.1009782. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34320039 Free PMC article.
-
Anti-inflammatory components of the Vietnamese starfish Protoreaster nodosus.Biol Res. 2015 Feb 20;48(1):12. doi: 10.1186/s40659-015-0002-2. Biol Res. 2015. PMID: 25762127 Free PMC article.
-
Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus.Infect Immun. 2016 Nov 18;84(12):3379-3387. doi: 10.1128/IAI.00185-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27620720 Free PMC article.
-
CCR7/dendritic cell axis mediates early bacterial dissemination in Orientia tsutsugamushi-infected mice.Front Immunol. 2022 Dec 22;13:1061031. doi: 10.3389/fimmu.2022.1061031. eCollection 2022. Front Immunol. 2022. PMID: 36618364 Free PMC article.
References
-
- Chi WC, Huang JJ, Sung JM, Lan RR, Ko WC, et al. Scrub typhus associated with multiorgan failure: A case report. Scand J Infect Dis. 1997;29:634–635. - PubMed
-
- Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from Thailand. Clin Infect Dis. 1994;18:624–626. - PubMed
-
- Murata M, Sudo K, Suzuki K, Aoyama Y, Nogami S, et al. Proliferating sites of Rickettsia tsutsugamushi in mice by different routes of inoculation evidenced with immunofluorescence. Jpn J Exp Med. 1985;55:193–199. - PubMed
-
- Ng FK, Oaks SC Jr Lee M, Groves MG, Lewis GE., Jr A scanning and transmission electron microscopic examination of rickettsia tsutsugamushi-infected human endothelial, MRC-5, and L-929 cells. Jpn J Med Sci Biol. 1985;38:125–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

