Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling
- PMID: 22723926
- PMCID: PMC3377598
- DOI: 10.1371/journal.pone.0039047
Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling
Abstract
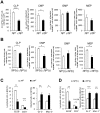
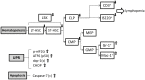
GRP78, a master regulator of the unfolded protein response (UPR) and cell signaling, is required for inner cell mass survival during early embryonic development. However, little is known about its role in adult hematopoietic stem cells (HSCs) and hematopoiesis. Here we generated a conditional knockout mouse model that acutely deletes Grp78 in the adult hematopoietic system. Acute GRP78 ablation resulted in a significant reduction of HSCs, common lymphoid and myeloid progenitors, and lymphoid cell populations in the mutant mice. The GRP78-null induced reduction of the HSC pool could be attributed to increased apoptosis. Chimeric mice with Grp78 deletion only in the hematopoietic cells also showed a loss of HSCs and lymphopenia, suggesting a cell intrinsic effect. Analysis of GRP78 deficient bone marrow (BM) cells showed constitutive activation of all the major UPR signaling pathways, including activation of eIF2α, ATF6, xbp-1 splicing, as well as caspase activation. A multiplex cytokine assay further revealed alteration in select cytokine and chemokine serum levels in the mutant mice. Collectively, these studies demonstrate that GRP78 plays a pleiotropic role in BM cells and contributes to HSC survival and the maintenance of the lymphoid lineage.
Conflict of interest statement
Figures




References
-
- Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002;9:606–612. - PubMed
-
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. - PubMed
-
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. - PubMed
-
- Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

