TYK2 kinase activity is required for functional type I interferon responses in vivo
- PMID: 22723949
- PMCID: PMC3377589
- DOI: 10.1371/journal.pone.0039141
TYK2 kinase activity is required for functional type I interferon responses in vivo
Abstract
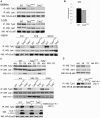
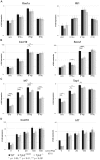
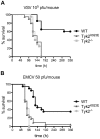
Tyrosine kinase 2 (TYK2) is a member of the Janus kinase (JAK) family and is involved in cytokine signalling. In vitro analyses suggest that TYK2 also has kinase-independent, i.e., non-canonical, functions. We have generated gene-targeted mice harbouring a mutation in the ATP-binding pocket of the kinase domain. The Tyk2 kinase-inactive (Tyk2(K923E)) mice are viable and show no gross abnormalities. We show that kinase-active TYK2 is required for full-fledged type I interferon- (IFN) induced activation of the transcription factors STAT1-4 and for the in vivo antiviral defence against viruses primarily controlled through type I IFN actions. In addition, TYK2 kinase activity was found to be required for the protein's stability. An inhibitory function was only observed upon over-expression of TYK2(K923E)in vitro. Tyk2(K923E) mice represent the first model for studying the kinase-independent function of a JAK in vivo and for assessing the consequences of side effects of JAK inhibitors.
Conflict of interest statement
Figures


Similar articles
-
The Atlantic salmon protein tyrosine kinase Tyk2: molecular cloning, modulation of expression and function.Dev Comp Immunol. 2013 Dec;41(4):553-63. doi: 10.1016/j.dci.2013.07.008. Epub 2013 Jul 18. Dev Comp Immunol. 2013. PMID: 23872231
-
JAK/STAT/SOCS-signaling pathway and colon and rectal cancer.Mol Carcinog. 2013 Feb;52(2):155-66. doi: 10.1002/mc.21841. Epub 2011 Nov 28. Mol Carcinog. 2013. PMID: 22121102 Free PMC article.
-
Structural and Functional Characterization of the JH2 Pseudokinase Domain of JAK Family Tyrosine Kinase 2 (TYK2).J Biol Chem. 2015 Nov 6;290(45):27261-27270. doi: 10.1074/jbc.M115.672048. Epub 2015 Sep 10. J Biol Chem. 2015. PMID: 26359499 Free PMC article.
-
Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity.Front Biosci (Landmark Ed). 2011 Jun 1;16(9):3214-32. doi: 10.2741/3908. Front Biosci (Landmark Ed). 2011. PMID: 21622231 Review.
-
Clinical Implications of Targeting the JAK-STAT Pathway in Psoriatic Disease: Emphasis on the TYK2 Pathway.J Cutan Med Surg. 2023 Jan-Feb;27(1_suppl):3S-24S. doi: 10.1177/12034754221141680. Epub 2022 Dec 15. J Cutan Med Surg. 2023. PMID: 36519621 Review.
Cited by
-
Non-Receptor Tyrosine Kinases: Their Structure and Mechanistic Role in Tumor Progression and Resistance.Cancers (Basel). 2024 Aug 2;16(15):2754. doi: 10.3390/cancers16152754. Cancers (Basel). 2024. PMID: 39123481 Free PMC article. Review.
-
Expression analysis of IFNAR1 and TYK2 transcripts in COVID-19 patients.Cytokine. 2022 May;153:155849. doi: 10.1016/j.cyto.2022.155849. Epub 2022 Mar 4. Cytokine. 2022. PMID: 35339044 Free PMC article.
-
TYK2 Variants in B-Acute Lymphoblastic Leukaemia.Genes (Basel). 2020 Nov 28;11(12):1434. doi: 10.3390/genes11121434. Genes (Basel). 2020. PMID: 33260630 Free PMC article. Clinical Trial.
-
TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis.J Clin Invest. 2020 Apr 1;130(4):1863-1878. doi: 10.1172/JCI126567. J Clin Invest. 2020. PMID: 32149730 Free PMC article.
-
Potential viral pathogenic mechanism in human type 1 diabetes.Diabetologia. 2014 Oct;57(10):2009-18. doi: 10.1007/s00125-014-3340-7. Epub 2014 Jul 30. Diabetologia. 2014. PMID: 25073445 Free PMC article. Review.
References
-
- Wilks AF. The JAK kinases: not just another kinase drug discovery target. Semin Cell Dev Biol. 2008;19:319–328. - PubMed
-
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. - PubMed
-
- Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. - PubMed
-
- Strobl B, Stoiber D, Sexl V, Müller M. Tyrosine kinase 2 (Tyk2) in cytokine signalling and host immunity. Front Biosci. 2011;17:3224–3232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

