Induction of monozygotic twinning by ascorbic acid in tobacco
- PMID: 22723952
- PMCID: PMC3377588
- DOI: 10.1371/journal.pone.0039147
Induction of monozygotic twinning by ascorbic acid in tobacco
Abstract
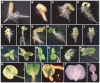
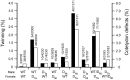
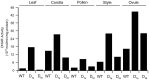
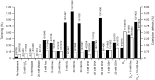
Embryo development in plants initiates following the transverse division of a zygote into an apical, proembryo cell and a basal cell that gives rise to the suspensor. Although mutants affected in embryo development through changes in cell division have been described, little is known about the control of the first zygotic division that gives rise to the proembryo. Ascorbic acid (Asc) promotes cell division by inducing G(1) to S progression but its role in embryo development has not been examined. In this study, we show that the level of dehydroascorbate reductase (DHAR) expression, which recycles Asc and regulates Asc pool size, affects the rate of monozygotic twinning and polycotyly. DHAR-induced twinning resulted from altered cell polarity and longitudinal instead of transverse cell division that generated embryos of equal size. Direct injection of Asc into ovaries phenocopied DHAR-induced twinning. Twinning induced by Asc was developmentally limited to the first two days after pollination whereas polycotyly was induced when the level of Asc was elevated just prior to cotyledon initiation. This work describes the first example of gene-directed monozygotic twinning and shows that Asc regulates cell polarity during embryo development.
Conflict of interest statement
Figures



References
-
- Coulter JM, Chamberlain CJ. In Morphology of Angiosperms. D. Appleton and Company, New York, New York. pp. 1903. pp. 212–226.
-
- Tisserat B, Esan BR, Murashige T. Somatic embryogenesis in angiosperms. Hort Rev. 1979;1:1B78.
-
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc. 1997;61:51–94.
-
- Vernon DM, Meinke DW. Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev Biol. 1994;165:566–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

