Genotype-specific differences between mouse CNS stem cell lines expressing frontotemporal dementia mutant or wild type human tau
- PMID: 22723997
- PMCID: PMC3377636
- DOI: 10.1371/journal.pone.0039328
Genotype-specific differences between mouse CNS stem cell lines expressing frontotemporal dementia mutant or wild type human tau
Abstract
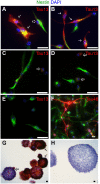
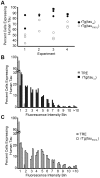
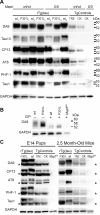
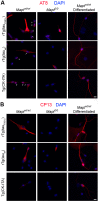
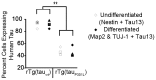
Stem cell (SC) lines that capture the genetics of disease susceptibility provide new research tools. To assess the utility of mouse central nervous system (CNS) SC-containing neurosphere cultures for studying heritable neurodegenerative disease, we compared neurosphere cultures from transgenic mice that express human tau with the P301L familial frontotemporal dementia (FTD) mutation, rTg(tau(P301L))4510, with those expressing comparable levels of wild type human tau, rTg(tau(wt))21221. rTg(tau(P301L))4510 mice express the human tau(P301L) variant in their forebrains and display cellular, histological, biochemical and behavioral abnormalities similar to those in human FTD, including age-dependent differences in tau phosphorylation that distinguish them from rTg(tau(wt))21221 mice. We compared FTD-hallmark tau phosphorylation in neurospheres from rTg(tau(P301L))4510 mice and from rTg(tau(wt))21221 mice. The tau genotype-specific phosphorylation patterns in neurospheres mimicked those seen in mice, validating use of neurosphere cultures as models for studying tau phosphorylation. Genotype-specific tau phosphorylation was observed in 35 independent cell lines from individual fetuses; tau in rTg(tau(P301L))4510 cultures was hypophosphorylated in comparison with rTg(tau(wt))21221 as was seen in young adult mice. In addition, there were fewer human tau-expressing cells in rTg(tau(P301L))4510 than in rTg(tau(wt))21221 cultures. Following differentiation, neuronal filopodia-spine density was slightly greater in rTg(tau(P301L))4510 than rTg(tau(wt))21221 and control cultures. Together with the recapitulation of genotype-specific phosphorylation patterns, the observation that neurosphere lines maintained their cell line-specific-differences and retained SC characteristics over several passages supports the utility of SC cultures as surrogates for analysis of cellular disease mechanisms.
Conflict of interest statement
Figures



Comment in
-
Editorial Note: Genotype-Specific Differences between Mouse CNS Stem Cell Lines Expressing Frontotemporal Dementia Mutant or Wild Type Human Tau.PLoS One. 2024 Jun 27;19(6):e0305843. doi: 10.1371/journal.pone.0305843. eCollection 2024. PLoS One. 2024. PMID: 38935762 Free PMC article. No abstract available.
References
-
- Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Urbach A, Schuldiner M, Benvenisty N. Modeling for Lesch-Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells. 2004;22:635–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

