Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial
- PMID: 22724029
- PMCID: PMC3378617
- DOI: 10.1371/journal.pntd.0001674
Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial
Abstract
Background: Alternative treatments for visceral leishmaniasis (VL) are required in East Africa. Paromomycin sulphate (PM) has been shown to be efficacious for VL treatment in India.
Methods: A multi-centre randomized-controlled trial (RCT) to compare efficacy and safety of PM (20 mg/kg/day for 21 days) and PM plus sodium stibogluconate (SSG) combination (PM, 15 mg/kg/day and SSG, 20 mg/kg/day for 17 days) with SSG (20 mg/kg/day for 30 days) for treatment of VL in East Africa. Patients aged 4-60 years with parasitologically confirmed VL were enrolled, excluding patients with contraindications. Primary and secondary efficacy outcomes were parasite clearance at 6-months follow-up and end of treatment, respectively. Safety was assessed mainly using adverse event (AE) data.
Findings: The PM versus SSG comparison enrolled 205 patients per arm with primary efficacy data available for 198 and 200 patients respectively. The SSG & PM versus SSG comparison enrolled 381 and 386 patients per arm respectively, with primary efficacy data available for 359 patients per arm. In Intention-to-Treat complete-case analyses, the efficacy of PM was significantly lower than SSG (84.3% versus 94.1%, difference = 9.7%, 95% confidence interval, CI: 3.6 to 15.7%, p = 0.002). The efficacy of SSG & PM was comparable to SSG (91.4% versus 93.9%, difference = 2.5%, 95% CI: -1.3 to 6.3%, p = 0.198). End of treatment efficacy results were very similar. There were no apparent differences in the safety profile of the three treatment regimens.
Conclusion: The 17 day SSG & PM combination treatment had a good safety profile and was similar in efficacy to the standard 30 day SSG treatment, suggesting suitability for VL treatment in East Africa.
Clinical trials registration: www.clinicaltrials.govNCT00255567.
Conflict of interest statement
The authors have read the journal's policy and have the following conflicts: Manica Balasegaram is employed by DNDi as Head of VL Clinical Program. Sally Ellis is employed by DNDi as Clinical Manager (VL). Robert Kimutai is employed by DNDi as a Clinical Trial Manager. Raymond Omollo works for DNDi. Marius Mueller works for MSF.
Figures
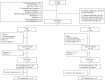

References
-
- Control of the Leishmaniases: Report of a meeting of the WHO expert committee on the control of Leishmaniases, Geneva, 22–26 March 2010. Switzerland: World Health Organization;
-
- World Health Organization. Fifth Consultative Meeting on Leishmania/HIV Coinfection, Addis Ababa, Ethiopia, 20–22 March2007. Switzerland: World Health Organization; 2007.
-
- Rijal S, Chappuis F, Singh R, Boelaert M, Loutan L, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Trans R Soc Trop Med Hyg. 2003;97:597–598. - PubMed
-
- Ritmeijer K, Dejenie A, Assefa Y, Hundie TB, Mesure J, et al. A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis. 2006;43:357–364. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

