Massively parallel sequencing of the mouse exome to accurately identify rare, induced mutations: an immediate source for thousands of new mouse models
- PMID: 22724066
- PMCID: PMC3376740
- DOI: 10.1098/rsob.120061
Massively parallel sequencing of the mouse exome to accurately identify rare, induced mutations: an immediate source for thousands of new mouse models
Abstract
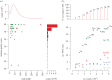
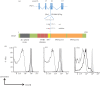
Accurate identification of sparse heterozygous single-nucleotide variants (SNVs) is a critical challenge for identifying the causative mutations in mouse genetic screens, human genetic diseases and cancer. When seeking to identify causal DNA variants that occur at such low rates, they are overwhelmed by false-positive calls that arise from a range of technical and biological sources. We describe a strategy using whole-exome capture, massively parallel DNA sequencing and computational analysis, which identifies with a low false-positive rate the majority of heterozygous and homozygous SNVs arising de novo with a frequency of one nucleotide substitution per megabase in progeny of N-ethyl-N-nitrosourea (ENU)-mutated C57BL/6j mice. We found that by applying a strategy of filtering raw SNV calls against known and platform-specific variants we could call true SNVs with a false-positive rate of 19.4 per cent and an estimated false-negative rate of 21.3 per cent. These error rates are small enough to enable calling a causative mutation from both homozygous and heterozygous candidate mutation lists with little or no further experimental validation. The efficacy of this approach is demonstrated by identifying the causative mutation in the Ptprc gene in a lymphocyte-deficient strain and in 11 other strains with immune disorders or obesity, without the need for meiotic mapping. Exome sequencing of first-generation mutant mice revealed hundreds of unphenotyped protein-changing mutations, 52 per cent of which are predicted to be deleterious, which now become available for breeding and experimental analysis. We show that exome sequencing data alone are sufficient to identify induced mutations. This approach transforms genetic screens in mice, establishes a general strategy for analysing rare DNA variants and opens up a large new source for experimental models of human disease.
Keywords: DNA capture; N-ethyl-N-nitrosourea mutagenesis; exome sequencing; mouse; mutation detection; variation detection.
Figures

References
-
- Acevedo-Arozena A, Wells S, Potter P, Kelly M, Cox RD, Brown SDM. 2008. ENU mutagenesis, a way forward to understand gene function. Annu. Rev. Genomics Hum. Genet. 9, 49–6910.1146/annurev.genom.9.081307.164224 (doi:10.1146/annurev.genom.9.081307.164224) - DOI - DOI - PubMed
-
- Justice MJ, Noveroske JK, Weber JS, Zheng B, Bradley A. 1999. Mouse ENU mutagenesis. Hum. Mol. Genet. 8, 1955–196310.1093/hmg/8.10.1955 (doi:10.1093/hmg/8.10.1955) - DOI - DOI - PubMed
-
- Albert TJ, et al. 2007. Direct selection of human genomic loci by microarray hybridization. Nat. Methods 4, 903–90510.1038/nmeth1111 (doi:10.1038/nmeth1111) - DOI - DOI - PubMed
-
- Gnirke A, et al. 2009. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat. Biotechnol. 27, 182–18910.1038/nbt.1523 (doi:10.1038/nbt.1523) - DOI - DOI - PMC - PubMed
-
- Ng SB, Nickerson DA, Bamshad MJ, Shendure J. 2010. Massively parallel sequencing and rare disease. Hum. Mol. Genet. 19, R119–R12410.1093/hmg/ddq390 (doi:10.1093/hmg/ddq390) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
